Why is Aged Acetylcholinesterase So Difficult to Reactivate?
- PMID: 28869561
- PMCID: PMC6151809
- DOI: 10.3390/molecules22091464
Why is Aged Acetylcholinesterase So Difficult to Reactivate?
Abstract
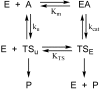
Organophosphorus agents are potent inhibitors of acetylcholinesterase. Inhibition involves successive chemical events. The first is phosphylation of the active site serine to produce a neutral adduct, which is a close structural analog of the acylation transition state. This adduct is unreactive toward spontaneous hydrolysis, but in many cases can be reactivated by nucleophilic medicinal agents, such as oximes. However, the initial phosphylation reaction may be followed by a dealkylation reaction of the incipient adduct. This reaction is called aging and produces an anionic phosphyl adduct with acetylcholinesterase that is refractory to reactivation. This review considers why the anionic aged adduct is unreactive toward nucleophiles. An alternate approach is to realkylate the aged adduct, which would render the adduct reactivatable with oxime nucleophiles. However, this approach confronts a considerable-and perhaps intractable-challenge: the aged adduct is a close analog of the deacylation transition state. Consequently, the evolutionary mechanisms that have led to transition state stabilization in acetylcholinesterase catalysis are discussed herein, as are the challenges that they present to reactivation of aged acetylcholinesterase.
Keywords: acetylcholinesterase; aging; inhibition; organophosphorus agent; reactivation; transition state.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

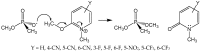
References
-
- Dingeman J., Jupa R. Chemical warfare in the Iran-Iraq conflict. Strategy Tactics. 1987;113:51–52.
-
- Tu A.T. Overview of sarin terrorist attacks in Japan. ACS Symp. Ser. 2000;745:304–317.
-
- Paddock R.C., Sang-Hun C. Kim Jong-nam was killed by VX nerve agent, Malaysians say. New York Times. Feb 23, 2017.
-
- Malany S., Sawai M., Sikorski R.S., Seravalli J., Quinn D.M., Radić Z., Taylor P., Kronman C., Velan B., Shafferman A. Transition state structure and rate determination for the acylation stage of acetylcholinesterase catalyzed hydrolysis of (acetylthio)choline. J. Am. Chem. Soc. 2000;122:2981–2987. doi: 10.1021/ja9933590. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

