PKR activation causes inflammation and MMP-13 secretion in human degenerated articular chondrocytes
- PMID: 28869834
- PMCID: PMC5582648
- DOI: 10.1016/j.redox.2017.08.011
PKR activation causes inflammation and MMP-13 secretion in human degenerated articular chondrocytes
Abstract
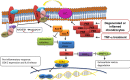
Osteoarthritis (OA) is a degenerative joint disease affecting a large population of people. Although the elevated expression of PKR (double stranded RNA-dependent protein kinase) and MMP-13 (collagenase-3) have been indicated to play pivotal roles in the pathogenesis of OA, the exact mechanism underlying the regulation of MMP-13 by PKR following inflammatory stimulation was relatively unknown. The purpose of this study was to determine the signaling pathway involved in the PKR-mediated induction of MMP-13 after TNF-α-stimulation. In this study, cartilages of knee joint were obtained from OA subjects who underwent arthroplastic knee surgery. Cartilages were used for tissue analysis or for chondrocytes isolation. In results, the upregulated expression of PKR was observed in damaged OA cartilages as well as in TNF-α-stimulated chondrocytes. Phosphorylation of PKC (protein kinase C) was found after TNF-α administration or PKR activation using poly(I:C), indicating PKC was regulated by PKR. The subsequent increased activity of NADPH oxidase led to oxidative stress accumulation and antioxidant capacity downregulation followed by an exaggerated inflammatory response with elevated levels of COX-2 and IL-8 via ERK/NF-κB pathway. Activated ERK pathway also impeded the inhibition of MMP-13 by PPAR-γ. These findings demonstrated that TNF-α-induced PKR activation triggered oxidative stress-mediated inflammation and MMP-13 in human chondrocytes. Unraveling these deregulated signaling cascades will deepen our knowledge of OA pathophysiology and provide aid in the development of novel therapies.
Keywords: Human chondrocyte; MMP-13; Osteoarthritis; Oxidative stress; PKR.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures








References
-
- Goldring S.R., Goldring M.B. Clinical aspects, pathology and pathophysiology of osteoarthritis. J. Musculoskelet. Neuron. Interact. 2006;6(4):376–378. - PubMed
-
- Goldring M.B., Goldring S.R. Osteoarthritis. J. Cell Physiol. 2007;213(3):626–634. - PubMed
-
- Malemud C.J., Islam N., Haqqi T.M. Pathophysiological mechanisms in osteoarthritis lead to novel therapeutic strategies. Cells Tissues Organs. 2003;174(1–2):34–48. - PubMed
-
- Aigner T., Fundel K., Saas J., Gebhard P.M., Haag J., Weiss T., Zien A., Obermayr F., Zimmer R., Bartnik E. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum. 2006;54(11):3533–3544. - PubMed
-
- Goldring M.B. Osteoarthritis and cartilage: the role of cytokines. Curr. Rheumatol. Rep. 2000;2(6):459–465. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

