Effects of Intensive Systolic Blood Pressure Control on Kidney and Cardiovascular Outcomes in Persons Without Kidney Disease: A Secondary Analysis of a Randomized Trial
- PMID: 28869987
- PMCID: PMC8545525
- DOI: 10.7326/M16-2966
Effects of Intensive Systolic Blood Pressure Control on Kidney and Cardiovascular Outcomes in Persons Without Kidney Disease: A Secondary Analysis of a Randomized Trial
Abstract
Background: The public health significance of the reported higher incidence of chronic kidney disease (CKD) with intensive systolic blood pressure (SBP) lowering is unclear.
Objective: To examine the effects of intensive SBP lowering on kidney and cardiovascular outcomes and contrast its apparent beneficial and adverse effects.
Design: Subgroup analyses of SPRINT (Systolic Blood Pressure Intervention Trial). (ClinicalTrials.gov: NCT01206062).
Setting: Adults with high blood pressure and elevated cardiovascular risk.
Participants: 6662 participants with a baseline estimated glomerular filtration rate (eGFR) of at least 60 mL/min/1.73 m2.
Intervention: Random assignment to an intensive or standard SBP goal (120 or 140 mm Hg, respectively).
Measurements: Differences in mean eGFR during follow-up (estimated with a linear mixed-effects model), prespecified incident CKD (defined as a >30% decrease in eGFR to a value <60 mL/min/1.73 m2), and a composite of all-cause death or cardiovascular event, with surveillance every 3 months.
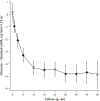
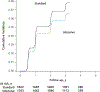
Results: The difference in adjusted mean eGFR between the intensive and standard groups was -3.32 mL/min/1.73 m2 (95% CI, -3.90 to -2.74 mL/min/1.73 m2) at 6 months, was -4.50 mL/min/1.73 m2 (CI, -5.16 to -3.85 mL/min/1.73 m2) at 18 months, and remained relatively stable thereafter. An incident CKD event occurred in 3.7% of participants in the intensive group and 1.0% in the standard group at 3-year follow-up, with a hazard ratio of 3.54 (CI, 2.50 to 5.02). The corresponding percentages for the composite of death or cardiovascular event were 4.9% and 7.1% at 3-year follow-up, with a hazard ratio of 0.71 (CI, 0.59 to 0.86).
Limitation: Long-term data were lacking.
Conclusion: Intensive SBP lowering increased risk for incident CKD events, but this was outweighed by cardiovascular and all-cause mortality benefits.
Primary funding source: National Institutes of Health.
Figures


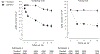


Comment in
-
More From SPRINT (Systolic Blood Pressure Intervention Trial): A Closer Look at the Price of Intensive Blood Pressure Control.Am J Kidney Dis. 2018 May;71(5):611-614. doi: 10.1053/j.ajkd.2017.11.008. Am J Kidney Dis. 2018. PMID: 29352606 No abstract available.
References
-
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427 - DOI - PubMed
-
- Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med. 2005;165:923–8. - PubMed
-
- Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383:1899–911. doi: 10.1016/S0140-6736(14)60685-1 - DOI - PMC - PubMed
-
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1 TR000433/TR/NCATS NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- UL1 TR000064/TR/NCATS NIH HHS/United States
- UL1 TR000075/TR/NCATS NIH HHS/United States
- UL1 TR000093/TR/NCATS NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- UL1 TR000050/TR/NCATS NIH HHS/United States
- UL1 RR025755/RR/NCRR NIH HHS/United States
- R01 DK091437/DK/NIDDK NIH HHS/United States
- M01 RR000042/RR/NCRR NIH HHS/United States
- HHSN268200900048C/HL/NHLBI NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- HHSN268200900040C/HL/NHLBI NIH HHS/United States
- HHSN268200900046C/HL/NHLBI NIH HHS/United States
- P30 GM103337/GM/NIGMS NIH HHS/United States
- UL1 TR001064/TR/NCATS NIH HHS/United States
- UL1 RR025752/RR/NCRR NIH HHS/United States
- UL1 RR025771/RR/NCRR NIH HHS/United States
- HHSN268200900049C/HL/NHLBI NIH HHS/United States
- HHSN268200900047C/HL/NHLBI NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UL1 TR000073/TR/NCATS NIH HHS/United States
- UL1 TR000002/TR/NCATS NIH HHS/United States
- UL1 TR000105/TR/NCATS NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- R21 DK106574/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
