Pessary or Progesterone to Prevent Preterm delivery in women with short cervical length: the Quadruple P randomised controlled trial
- PMID: 28870155
- PMCID: PMC5584011
- DOI: 10.1186/s12884-017-1454-x
Pessary or Progesterone to Prevent Preterm delivery in women with short cervical length: the Quadruple P randomised controlled trial
Abstract
Background: Preterm birth is in quantity and in severity the most important topic in obstetric care in the developed world. Progestogens and cervical pessaries have been studied as potential preventive treatments with conflicting results. So far, no study has compared both treatments.
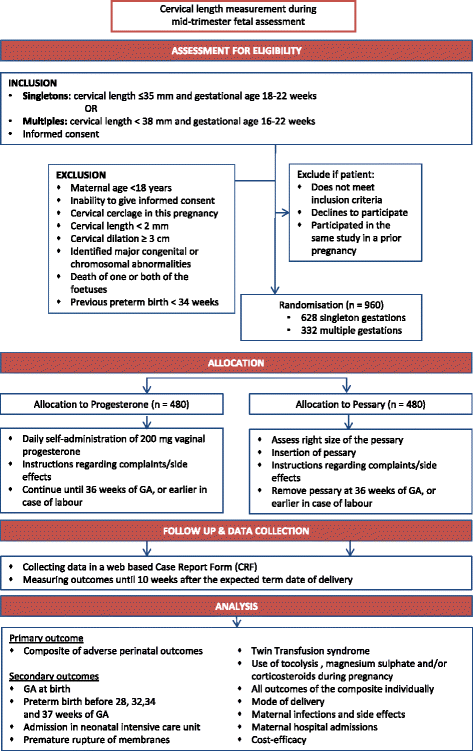
Methods/design: The Quadruple P study aims to compare the efficacy of vaginal progesterone and cervical pessary in the prevention of adverse perinatal outcome associated with preterm birth in asymptomatic women with a short cervix, in singleton and multiple pregnancies separately. It is a nationwide open-label multicentre randomized clinical trial (RCT) with a superiority design and will be accompanied by an economic analysis. Pregnant women undergoing the routine anomaly scan will be offered cervical length measurement between 18 and 22 weeks in a singleton and at 16-22 weeks in a multiple pregnancy. Women with a short cervix, defined as less than, or equal to 35 mm in a singleton and less than 38 mm in a multiple pregnancy, will be invited to participate in the study. Eligible women will be randomly allocated to receive either progesterone or a cervical pessary. Following randomization, the silicone cervical pessary will be placed during vaginal examination or 200 mg progesterone capsules will be daily self-administered vaginally. Both interventions will be continued until 36 weeks gestation or until delivery, whichever comes first. Primary outcome will be composite adverse perinatal outcome of perinatal mortality and perinatal morbidity including bronchopulmonary dysplasia, intraventricular haemorrhage grade III and IV, periventricular leukomalacia higher than grade I, necrotizing enterocolitis higher than stage I, Retinopathy of prematurity (ROP) or culture proven sepsis. These outcomes will be measured up until 10 weeks after the expected due date. Secondary outcomes will be, among others, time to delivery, preterm birth rate before 28, 32, 34 and 37 weeks, admission to neonatal intensive care unit, maternal morbidity, maternal admission days for threatened preterm labour and costs.
Discussion: This trial will provide evidence on whether vaginal progesterone or a cervical pessary is more effective in decreasing adverse perinatal outcome in both singletons and multiples.
Trial registration: Trial registration number: NTR 4414 . Date of registration January 29th 2014.
Keywords: Multiples; Pessary; Preterm birth; Prevention; Progesterone; Singletons.
Conflict of interest statement
Ethics approval and consent to participate
The Medical Ethics Committee of the Academic Medical Centre Amsterdam has approved the study (MEC AMC 2013_019). The trial is registered in the Dutch Trial register (Trial registration number: NTR 4414. Date of registration January 29th 2014). See
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests..
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Perinatale Zorg in Nederland. 2015. https://www.perined.nl/producten/publicaties/jaarboeken. Accessed 10 May 2017.
-
- Lim AC, Bloemenkamp KW, Boer K, Duvekot JJ, Erwich JJ, Hasaart TH, Hummel P, Mol BW, Offermans JP, van Oirschot CM, et al. Progesterone for the prevention of preterm birth in women with multiple pregnancies: the AMPHIA trial. BMC Pregnancy Childbirth. 2007;7:7. doi: 10.1186/1471-2393-7-7. - DOI - PMC - PubMed
-
- Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371(9607):164–175. doi:10.1016/S0140-6736(08)60108-7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical