Obstetric professionals' perceptions of non-invasive prenatal testing for Down syndrome: clinical usefulness compared with existing tests and ethical implications
- PMID: 28870159
- PMCID: PMC5583989
- DOI: 10.1186/s12884-017-1474-6
Obstetric professionals' perceptions of non-invasive prenatal testing for Down syndrome: clinical usefulness compared with existing tests and ethical implications
Abstract
Background: While non-invasive prenatal testing (NIPT) for fetal aneuploidy is commercially available in many countries, little is known about how obstetric professionals in non-Western populations perceive the clinical usefulness of NIPT in comparison with existing first-trimester combined screening (FTS) for Down syndrome (DS) or invasive prenatal diagnosis (IPD), or perceptions of their ethical concerns arising from the use of NIPT.
Methods: A cross-sectional survey among 327 obstetric professionals (237 midwives, 90 obstetricians) in Hong Kong.
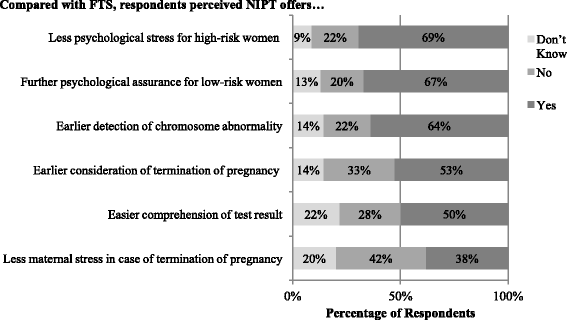
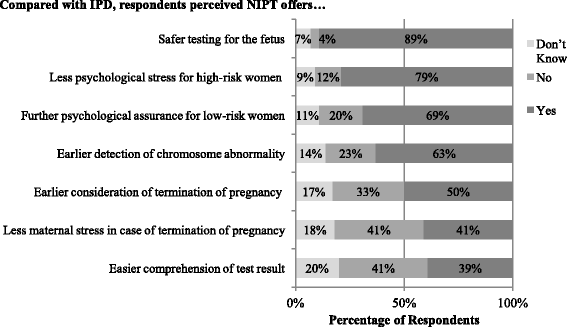
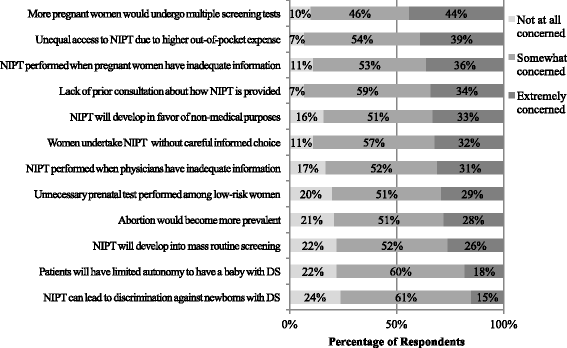
Results: Compared to FTS, NIPT was believed to: provide more psychological benefits and enable earlier consideration of termination of pregnancy. Compared to IPD, NIPT was believed to: provide less psychological stress for high-risk women and more psychological assurance for low-risk women, and offer an advantage to detect chromosomal abnormalities earlier. Significant differences in perceived clinical usefulness were found by profession and healthcare sector: (1) obstetricians reported more certain views towards the usefulness of NIPT than midwives and (2) professionals in the public sector perceived less usefulness of NIPT than the private sector. Beliefs about earlier detection of DS using NIPT were associated with ethical concerns about increasing abortion. Participants believing that NIPT provided psychological assurance among low-risk women were less likely to be concerned about ethical issues relating to informed decision-making and pre-test consultation for NIPT.
Conclusions: Our findings suggest the need for political debate initially on how to ensure pregnant women accessing public services are informed about commercially available more advanced technology, but also on the potential implementation of NIPT within public services to improve access and equity to DS screening services.
Keywords: Attitude; Cell-free fetal DNA; Clinical decision-making; Down syndrome; Ethical concern; Hong Kong; Informed consent; Non-invasive prenatal test.
Conflict of interest statement
Ethics approval and consent to participate
The Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee approved this study. A written informed consent was obtained from all survey respondents to participate in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

