Effect of maternal vitamin D3 supplementation on maternal health, birth outcomes, and infant growth among HIV-infected Tanzanian pregnant women: study protocol for a randomized controlled trial
- PMID: 28870263
- PMCID: PMC5584035
- DOI: 10.1186/s13063-017-2157-3
Effect of maternal vitamin D3 supplementation on maternal health, birth outcomes, and infant growth among HIV-infected Tanzanian pregnant women: study protocol for a randomized controlled trial
Abstract
Background: Vitamin D has significant immunomodulatory effects on both adaptive and innate immune responses. Observational studies indicate that adults infected with HIV with low vitamin D status may be at increased risk of mortality, pulmonary tuberculosis, and HIV disease progression. Growing observational evidence also suggests that low vitamin D status in pregnancy may increase the risk of adverse birth and infant health outcomes. As a result, antiretroviral therapy (ART) adjunct vitamin D3 supplementation may improve the health of HIV-infected pregnant women and their children.
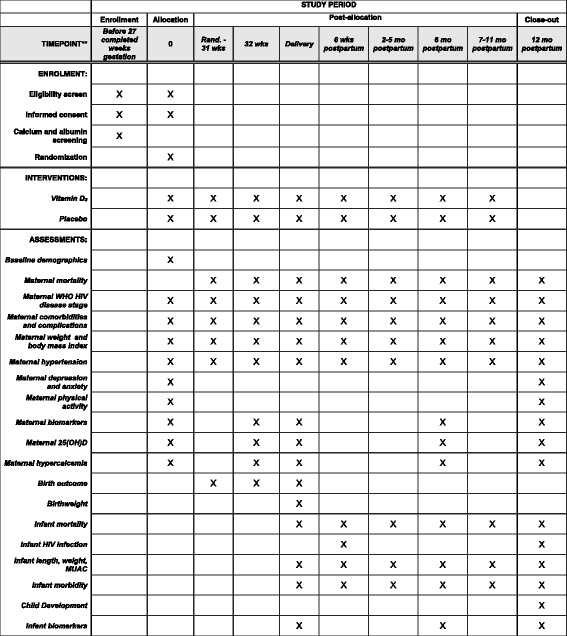
Methods/design: The Trial of Vitamins-5 (ToV5) is an individually randomized, double-blind, placebo-controlled trial of maternal vitamin D3 (cholecalciferol) supplementation conducted among 2300 HIV-infected pregnant women receiving triple-drug ART under Option B+ in Dar es Salaam, Tanzania. HIV-infected pregnant women of 12-27 weeks gestation are randomized to either: 1) 3000 IU vitamin D3 taken daily from randomization in pregnancy until trial discharge at 12 months postpartum; or 2) a matching placebo regimen. Maternal participants are followed-up at monthly clinic visits during pregnancy, at delivery, and then with their children at monthly postpartum clinic visits. The primary efficacy outcomes of the trial are: 1) maternal HIV disease progression or death; 2) risk of small-for-gestational age (SGA) births; and 3) risk of infant stunting at 1 year of age. The primary safety outcome of the trial is incident maternal hypercalcemia. Secondary outcomes include a range of clinical and biological maternal and child health outcomes.
Discussion: The ToV5 will provide causal evidence on the effect of vitamin D3 supplementation on HIV progression and death, SGA births, and infant stunting at 1 year of age. The results of the trial are likely generalizable to HIV-infected pregnant women and their children in similar resource-limited settings utilizing the Option B+ approach.
Trial registration: ClinicalTrials.gov identifier: NCT02305927 . Registered on 29 October 2014.
Conflict of interest statement
Ethics approval and consent to participate
The ToV5 protocol was approved by the Harvard T.H. Chan School of Public Health Institutional Review Board (reference no. IRB14-3416), the Tanzanian National Health Research Ethics Sub-Committee (NatHREC; reference no. NIMR/HQ/R.8a/Vol.IX/1933), and the Tanzania Food and Drug Authority (TFDA; reference no. TFDA15/CTR/0003/5). All participants will provide written informed consent for both screening and trial enrollment. Individuals who consent for trial enrollment are also asked for consent to escort the participant home to register the address, to contact the participant, neighbors, or relatives by cell phone or a home visit if the participant misses a clinic visit, and to store and use of all data and maternal and child blood samples for future research studies.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz C, Hayani K, Handelsman E, Smeriglio V, Hoff R, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29(5):484–94. doi: 10.1097/00042560-200204150-00009. - DOI - PubMed
-
- World Health Organization . Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Switzerland: World Health Organization; 2015. - PubMed
-
- World Health Organization . Consolidated guidelines on general HIV care and the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, Switzerland: World Health Organization; 2013.
-
- UNAIDS . Global plan towards the elimination of new HIV infections among children by 2015. Geneva, Switzerland: UNAIDS; 2011.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials