Maternal Dead-end 1 promotes translation of nanos1 by binding the eIF3 complex
- PMID: 28870987
- PMCID: PMC5675448
- DOI: 10.1242/dev.152611
Maternal Dead-end 1 promotes translation of nanos1 by binding the eIF3 complex
Abstract
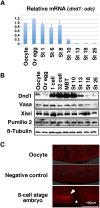
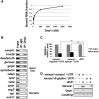
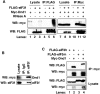
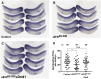
In the developing embryo, primordial germ cells (PGCs) represent the exclusive progenitors of the gametes, and their loss results in adult infertility. During early development, PGCs are exposed to numerous signals that specify somatic cell fates. To prevent somatic differentiation, PGCs must transiently silence their genome, an early developmental process that requires Nanos activity. However, it is unclear how Nanos translation is regulated in developing embryos. We report here that translation of nanos1 after fertilization requires Dead-end 1 (Dnd1), a vertebrate-specific germline RNA-binding protein. We provide evidence that Dnd1 protein, expression of which is low in oocytes, but increases dramatically after fertilization, directly interacts with, and relieves the inhibitory function of eukaryotic initiation factor 3f, a repressive component in the 43S preinitiation complex. This work uncovers a novel translational regulatory mechanism that is fundamentally important for germline development.
Keywords: Dnd1; Germline development; Nanos; Translation regulation; Xenopus.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

