Three-dimensional automated reporter quantification (3D-ARQ) technology enables quantitative screening in retinal organoids
- PMID: 28870990
- PMCID: PMC5675442
- DOI: 10.1242/dev.146290
Three-dimensional automated reporter quantification (3D-ARQ) technology enables quantitative screening in retinal organoids
Abstract
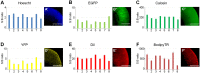
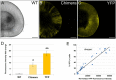
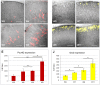
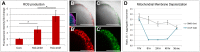
The advent of stem cell-derived retinal organoids has brought forth unprecedented opportunities for developmental and physiological studies, while presenting new therapeutic promise for retinal degenerative diseases. From a translational perspective, organoid systems provide exciting new prospects for drug discovery, offering the possibility to perform compound screening in a three-dimensional (3D) human tissue context that resembles the native histoarchitecture and to some extent recapitulates cellular interactions. However, inherent variability issues and a general lack of robust quantitative technologies for analyzing organoids on a large scale pose severe limitations for their use in translational applications. To address this need, we have developed a screening platform that enables accurate quantification of fluorescent reporters in complex human iPSC-derived retinal organoids. This platform incorporates a fluorescence microplate reader that allows xyz-dimensional detection and fine-tuned wavelength selection. We have established optimal parameters for fluorescent reporter signal detection, devised methods to compensate for organoid size variability, evaluated performance and sensitivity parameters, and validated this technology for functional applications.
Keywords: 3D-ARQ; Fluorescence reporter quantification; Human; Retinal organoids; Screening.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
-
- Burridge P. W., Thompson S., Millrod M. A., Weinberg S., Yuan X., Peters A., Mahairaki V., Koliatsos V. E., Tung L. and Zambidis E. T. (2011). A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS ONE 6, e18293 10.1371/journal.pone.0018293 - DOI - PMC - PubMed
-
- Cano M., Thimmalappula R., Fujihara M., Nagai N., Sporn M., Wang A. L., Neufeld A. H., Biswal S. and Handa J. T. (2010). Cigarette smoking, oxidative stress, the anti-oxidant response through Nrf2 signaling, and age-related macular degeneration. Vision Res. 50, 652-664. 10.1016/j.visres.2009.08.018 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

