Effectiveness of two psychological interventions for pain management, emotional regulation and promotion of quality of life among adult Portuguese men with haemophilia (PSY-HaEMOPEQ): study protocol for a single-centre prospective randomised controlled trial
- PMID: 28871021
- PMCID: PMC5588949
- DOI: 10.1136/bmjopen-2017-016973
Effectiveness of two psychological interventions for pain management, emotional regulation and promotion of quality of life among adult Portuguese men with haemophilia (PSY-HaEMOPEQ): study protocol for a single-centre prospective randomised controlled trial
Abstract
Introduction: Haemophilia is a bleeding disorder associated with significant pain, emotional distress, quality of life (QoL) impairment and considerable healthcare costs. Psychosocial health and effective pain management are considered essential end points for optimal haemophilia care, but there is a significant gap in evidence-based treatments targeting these outcomes in people with haemophilia (PWH). Psychological interventions are cost-effective in promoting emotional well-being, QoL and pain control, although these have been scarcely used in haemophilia field. This investigation aims to evaluate the effectiveness of two psychological interventions for pain management, emotional regulation and promotion of QoL in PWH.
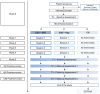
Methods and analysis: This is a single-centre parallel randomised controlled trial conducted at a European Haemophilia Comprehensive Care Centre in Portugal, with five assessment points: baseline (T0), postintervention (T1), 3 (T2), 6 (T3) and 12 (T4) months follow-up. Eligible adult males, with moderate or severe haemophilia A or B will be randomised to experimental (EG) or control (CG) group. Intervention is either cognitive-behavioural therapy (EG1) or hypnosis (EG2), both consisting of four weekly sessions following standardised scripts delivered by trained psychologists. Randomisation will be computer generated, allocation concealment will be guaranteed and outcome assessors will be blind to EG/CG allocation. Main outcomes are pain and haemophilia-related QoL and secondary outcomes include clinical (clotting factor replacement consumption, joint bleeding episodes, analgesic intake) and psychological (pain coping strategies, anxiety, depression, illness perceptions) variables, functional assessment of the joints, inflammatory biomarkers (cytokines, high-sensitivity C reactive protein) and white blood cell count.
Ethics and dissemination: This study was approved by the competent authorities and all procedures will comply with international ethical guidelines for clinical studies involving humans. Written informed consent will be obtained from all participants. The dissemination plan includes peer-reviewed scientific publications, conference participation and web and media coverage.
Trial registration number: NCT02870452.
Keywords: emotional well-being; haemophilia; health psychology; pain management; quality of life; randomized controled trial.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous