KLF9 and JNK3 Interact to Suppress Axon Regeneration in the Adult CNS
- PMID: 28871032
- PMCID: PMC5628408
- DOI: 10.1523/JNEUROSCI.0643-16.2017
KLF9 and JNK3 Interact to Suppress Axon Regeneration in the Adult CNS
Abstract
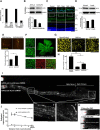
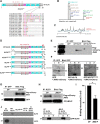
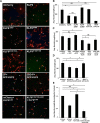
Neurons in the adult mammalian CNS decrease in intrinsic axon growth capacity during development in concert with changes in Krüppel-like transcription factors (KLFs). KLFs regulate axon growth in CNS neurons including retinal ganglion cells (RGCs). Here, we found that knock-down of KLF9, an axon growth suppressor that is normally upregulated 250-fold in RGC development, promotes long-distance optic nerve regeneration in adult rats of both sexes. We identified a novel binding partner, MAPK10/JNK3 kinase, and found that JNK3 (c-Jun N-terminal kinase 3) is critical for KLF9's axon-growth-suppressive activity. Interfering with a JNK3-binding domain or mutating two newly discovered serine phosphorylation acceptor sites, Ser106 and Ser110, effectively abolished KLF9's neurite growth suppression in vitro and promoted axon regeneration in vivo These findings demonstrate a novel, physiologic role for the interaction of KLF9 and JNK3 in regenerative failure in the optic nerve and suggest new therapeutic strategies to promote axon regeneration in the adult CNS.SIGNIFICANCE STATEMENT Injured CNS nerves fail to regenerate spontaneously. Promoting intrinsic axon growth capacity has been a major challenge in the field. Here, we demonstrate that knocking down Krüppel-like transcription factor 9 (KLF9) via shRNA promotes long-distance axon regeneration after optic nerve injury and uncover a novel and important KLF9-JNK3 interaction that contributes to axon growth suppression in vitro and regenerative failure in vivo These studies suggest potential therapeutic approaches to promote axon regeneration in injury and other degenerative diseases in the adult CNS.
Keywords: Jnk; KLFs; regeneration; survival.
Copyright © 2017 the authors 0270-6474/17/379632-13$15.00/0.
Figures



References
-
- Blackmore MG, Wang Z, Lerch JK, Motti D, Zhang YP, Shields CB, Lee JK, Goldberg JL, Lemmon VP, Bixby JL (2012) Krüppel-like Factor 7 engineered for transcriptional activation promotes axon regeneration in the adult corticospinal tract. Proc Natl Acad Sci U S A 109:7517–7522. 10.1073/pnas.1120684109 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
