Small molecule T63 suppresses osteoporosis by modulating osteoblast differentiation via BMP and WNT signaling pathways
- PMID: 28871136
- PMCID: PMC5583318
- DOI: 10.1038/s41598-017-10929-3
Small molecule T63 suppresses osteoporosis by modulating osteoblast differentiation via BMP and WNT signaling pathways
Abstract
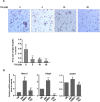
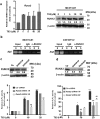
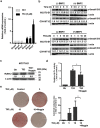
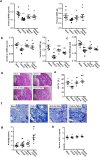
Osteoporosis results from the imbalance between bone resorption and bone formation, and restoring the normal balance of bone remodeling is highly desirable for identification of better treatment. In this study, using a cell-based high-throughput screening model representing Runt-related transcription factor 2 (RUNX2) transcriptional activity, we identified a novel small-molecular-weight compound, T63, as an efficient up-regulator of osteogenesis. T63 increased the alkaline phosphatase (ALPL) activity and mineralization as well as gene expression of Alpl and other osteogenic marker genes in mouse osteoblasts and mesenchymal stem cell-like cells. Upon induction of osteoblast differentiation, T63 inhibited adipogenic differentiation in the pluripotent mesenchymal cells. Consistently, T63 up-regulated RUNX2 mRNA and protein levels, and knockdown of RUNX2 reduced the osteogenic role of T63. Mechanistically, T63 activated both BMPs and WNT/β-catenin signaling pathways. Inhibition of either signaling pathway with specific inhibitor suppressed T63-induced RUNX2 expression and the osteogenic phenotypes. Moreover, T63 markedly protected against bone mass loss in the ovariectomized and dexamethasone treated rat osteoporosis model. Collectively, our data demonstrate that T63 could be a promising drug candidate and deserves further development for potential therapeutics in osteoporosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

