Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma
- PMID: 28871203
- PMCID: PMC5583399
- DOI: 10.1038/s41598-017-11220-1
Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma
Abstract
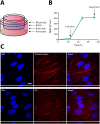
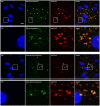
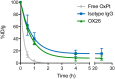
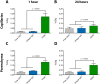
Drug delivery to the brain is hampered by the presence of the blood-brain barrier, which excludes most molecules from freely diffusing into the brain, and tightly regulates the active transport mechanisms that ensure sufficient delivery of nutrients to the brain parenchyma. Harnessing the possibility of delivering neuroactive drugs by way of receptors already present on the brain endothelium has been of interest for many years. The transferrin receptor is of special interest since its expression is limited to the endothelium of the brain as opposed to peripheral endothelium. Here, we investigate the possibility of delivering immunoliposomes and their encapsulated cargo to the brain via targeting of the transferrin receptor. We find that transferrin receptor-targeting increases the association between the immunoliposomes and primary endothelial cells in vitro, but that this does not correlate with increased cargo transcytosis. Furthermore, we show that the transferrin receptor-targeted immunoliposomes accumulate along the microvessels of the brains of rats, but find no evidence for transcytosis of the immunoliposome. Conversely, the increased accumulation correlated both with increased cargo uptake in the brain endothelium and subsequent cargo transport into the brain. These findings suggest that transferrin receptor-targeting is a relevant strategy of increasing drug exposure to the brain.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

