Single-Domain Antibodies As Versatile Affinity Reagents for Analytical and Diagnostic Applications
- PMID: 28871254
- PMCID: PMC5566570
- DOI: 10.3389/fimmu.2017.00977
Single-Domain Antibodies As Versatile Affinity Reagents for Analytical and Diagnostic Applications
Abstract
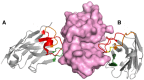
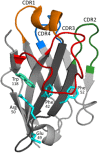
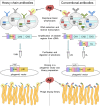
With just three CDRs in their variable domains, the antigen-binding site of camelid heavy-chain-only antibodies (HcAbs) has a more limited structural diversity than that of conventional antibodies. Even so, this does not seem to limit their specificity and high affinity as HcAbs against a broad range of structurally diverse antigens have been reported. The recombinant form of their variable domain [nanobody (Nb)] has outstanding properties that make Nbs, not just an alternative option to conventional antibodies, but in many cases, these properties allow them to reach analytical or diagnostic performances that cannot be accomplished with conventional antibodies. These attributes include comprehensive representation of the immune specificity in display libraries, easy adaptation to high-throughput screening, exceptional stability, minimal size, and versatility as affinity building block. Here, we critically reviewed each of these properties and highlight their relevance with regard to recent developments in different fields of immunosensing applications.
Keywords: VHH; haptens; imaging; immunodetection; nanobodies; phage display.
Figures

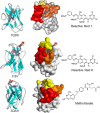
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

