Hops (Humulus lupulus L.) Bitter Acids: Modulation of Rumen Fermentation and Potential As an Alternative Growth Promoter
- PMID: 28871284
- PMCID: PMC5566628
- DOI: 10.3389/fvets.2017.00131
Hops (Humulus lupulus L.) Bitter Acids: Modulation of Rumen Fermentation and Potential As an Alternative Growth Promoter
Abstract
Antibiotics can improve ruminant growth and efficiency by altering rumen fermentation via selective inhibition of microorganisms. However, antibiotic use is increasingly restricted due to concerns about the spread of antibiotic-resistance. Plant-based antimicrobials are alternatives to antibiotics in animal production. The hops plant (Humulus lupulus L.) produces a range of bioactive secondary metabolites, including antimicrobial prenylated phloroglucinols, which are commonly called alpha- and beta-acids. These latter compounds can be considered phyto-ionophores, phytochemicals with a similar antimicrobial mechanism of action to ionophore antibiotics (e.g., monensin, lasalocid). Like ionophores, the hop beta-acids inhibit rumen bacteria possessing a classical Gram-positive cell envelope. This selective inhibition causes several effects on rumen fermentation that are beneficial to finishing cattle, such as decreased proteolysis, ammonia production, acetate: propionate ratio, and methane production. This article reviews the effects of hops and hop secondary metabolites on rumen fermentation, including the physiological mechanisms on specific rumen microorganisms, and consequences for the ruminant host and ruminant production. Further, we propose that hop beta-acids are useful model natural products for ruminants because of (1) the ionophore-like mechanism of action and spectrum of activity and (2) the literature available on the plant due to its use in brewing.
Keywords: alternatives to antibiotics; antimicrobial growth promoter; feed efficiency; phytochemicals; plant secondary metabolites; rumen microbiology.
Figures
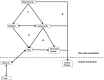

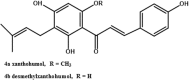



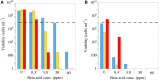

References
-
- Hungate RE. The Rumen and Its Microbes. New York: Academic Press; (1966).
-
- Allen MS. Drives and limits to feed intake in ruminants. Anim Prod Sci (2014) 54:1513–24. 10.1071/AN14478 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

