ALK1 signaling in development and disease: new paradigms
- PMID: 28871312
- PMCID: PMC5687069
- DOI: 10.1007/s00018-017-2636-4
ALK1 signaling in development and disease: new paradigms
Abstract
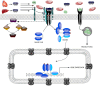
Activin A receptor like type 1 (ALK1) is a transmembrane serine/threonine receptor kinase in the transforming growth factor-beta receptor family that is expressed on endothelial cells. Defects in ALK1 signaling cause the autosomal dominant vascular disorder, hereditary hemorrhagic telangiectasia (HHT), which is characterized by development of direct connections between arteries and veins, or arteriovenous malformations (AVMs). Although previous studies have implicated ALK1 in various aspects of sprouting angiogenesis, including tip/stalk cell selection, migration, and proliferation, recent work suggests an intriguing role for ALK1 in transducing a flow-based signal that governs directed endothelial cell migration within patent, perfused vessels. In this review, we present an updated view of the mechanism of ALK1 signaling, put forth a unified hypothesis to explain the cellular missteps that lead to AVMs associated with ALK1 deficiency, and discuss emerging roles for ALK1 signaling in diseases beyond HHT.
Keywords: Activin A receptor like type 1; Angiogenesis; Arteriovenous malformation; Bone morphogenetic protein; Endoglin; Hereditary hemorrhagic telangiectasia.
Figures


References
-
- Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M, Pericak-Vance MA, Diamond A, Guttmacher AE, Jackson CE, Attisano L, Kucherlapati R, Porteous ME, Marchuk DA. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet. 1996;13:189–195. doi: 10.1038/ng0696-189. - DOI - PubMed
-
- McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J, McCormick MK, Pericak-Vance MA, Heutnik P, Oostra BA, Haitjema T, Westerman CJJ, Porteous ME, Guttmacher AE, Letarte M, Marchuk DA. Endoglin, a TGF-b binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–351. doi: 10.1038/ng1294-345. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

