Zoledronate dysregulates fatty acid metabolism in renal tubular epithelial cells to induce nephrotoxicity
- PMID: 28871336
- PMCID: PMC5773652
- DOI: 10.1007/s00204-017-2048-0
Zoledronate dysregulates fatty acid metabolism in renal tubular epithelial cells to induce nephrotoxicity
Abstract
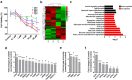
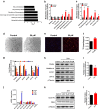
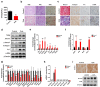
Zoledronate is a bisphosphonate that is widely used in the treatment of metabolic bone diseases. However, zoledronate induces significant nephrotoxicity associated with acute tubular necrosis and renal fibrosis when administered intravenously. There is speculation that zoledronate-induced nephrotoxicity may result from its pharmacological activity as an inhibitor of the mevalonate pathway but the molecular mechanisms are not fully understood. In this report, human proximal tubular HK-2 cells and mouse models were combined to dissect the molecular pathways underlying nephropathy caused by zoledronate treatments. Metabolomic and proteomic assays revealed that multiple cellular processes were significantly disrupted, including the TGFβ pathway, fatty acid metabolism and small GTPase signaling in zoledronate-treated HK-2 cells (50 μM) as compared with those in controls. Zoledronate treatments in cells (50 μM) and mice (3 mg/kg) increased TGFβ/Smad3 pathway activation to induce fibrosis and kidney injury, and specifically elevated lipid accumulation and expression of fibrotic proteins. Conversely, fatty acid transport protein Slc27a2 deficiency or co-administration of PPARA agonist fenofibrate (20 mg/kg) prevented zoledronate-induced lipid accumulation and kidney fibrosis in mice, indicating that over-expression of fatty acid transporter SLC27A2 and defective fatty acid β-oxidation following zoledronate treatments were significant factors contributing to its nephrotoxicity. These pharmacological and genetic studies provide an important mechanistic insight into zoledronate-associated kidney toxicity that will aid in development of therapeutic prevention and treatment options for this nephropathy.
Keywords: Fatty acid transporter; Lipid accumulation; Renal fibrosis; TGFβ1 signaling; Zoledronate.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical standards
The manuscript does not contain clinical studies or participant data.
Figures




References
-
- Bottinger EP, Bitzer M. TGF-β signaling in renal disease. J Am Soc Nephrol. 2002;13(10):2600–2610. doi: 10.1097/01.ASN.0000033611.79556.AE. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

