Prevalence and outcomes of HIV-1 diagnostic challenges during universal birth testing - an urban South African observational cohort
- PMID: 28872276
- PMCID: PMC6192462
- DOI: 10.7448/IAS.20.7.21761
Prevalence and outcomes of HIV-1 diagnostic challenges during universal birth testing - an urban South African observational cohort
Abstract
Introduction: HIV-1 polymerase chain reaction (PCR) testing at birth aims to facilitate earlier initiation of antiretroviral therapy (ART) for HIV-infected neonates. Data from two years of universal birth testing implementation in a high-burden South African urban setting are presented to demonstrate the prevalence and outcomes of diagnostic challenges in this context.
Methods: HIV-exposed neonates born at Rahima Moosa Mother and Child Hospital between 5 June 2014 and 31 August 2016 were routinely screened at birth for HIV-1 on whole blood samples using the COBAS® AmpliPrep/COBAS® TaqMan (CAP/CTM) HIV-1 Qualitative Test, version 2.0 (Roche Molecular Systems, Inc., Branchburg, NJ, USA). Virological results were interpreted according to standard operating procedures with the South African National Health Laboratory Service. All neonates with non-negative results were actively followed-up and categorized according to HIV infection status as positive, negative, uncertain and lost to follow-up (LTFU).
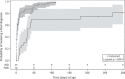
Results: 104 (1.8%) of 5743 HIV-exposed neonates received a non-negative birth PCR result, for which laboratory data were available for 102 (98%) cases - 78 (76%) tested positive and 24 (24%) indeterminate. HIV infection status was confirmed positive in 83 (81%) infants, negative in 8 (8%), uncertain in 5 (5%) and LTFU in 6 (6%) cases. The positive predictive value (excluding cases of uncertain diagnosis and inadequate testing) following a non-negative HIV-1 PCR screening test at birth was 0.91 (83/91; 95% confidence interval: 0.85-0.96). Neonates testing positive at birth had significantly higher viral load (VL) results than those testing indeterminate at birth of 4.5 and 3.0 log copies/ml (p = 0.0007), respectively. Similarly, mothers of neonates with positive as compared to indeterminate birth test results had higher VLs of 4.5 and 2.7 log copies/ml (p = 0.0013), respectively. Half of neonates with an indeterminate birth test were shown to be HIV-infected on subsequent confirmatory testing, with time to final diagnosis 30 days longer for these neonates (p < 0.0001).
Conclusion: Indeterminate HIV-1 PCR results accounted for a quarter of non-negative results at birth and were associated with a high risk of infection in comparison to the risk of in utero transmission. Indeterminate birth results with positive HIV PCR results on repeat testing were associated with later final diagnosis. The HIV-1 status remains uncertain in a minority of cases because of repeatedly indeterminate results, highlighting the need for more sensitive and specific virological tests.
Keywords: HIV-1 PCR; birth testing; early infant diagnosis; indeterminate.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


References
-
- National Department of Health . National consolidated guidelines for the prevention of mother‐to‐child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults edn 2015. Pretoria: National Department of Health; 2015.
-
- World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection ‐ recommendations for a public health approach. 2nd ed. Geneva: World Health Organization; 2016. - PubMed
-
- National Department of Health . The South African antiretroviral treatment guidelines 2013 edn 2013. Pretoria: National Department of Health; 2013.
-
- Sherman GG. Testing at birth – update from South Africa. Plenary presented at: 8th HIV Pediatric Workshop; 2016. 15–16 July; Durban, South Africa; 2016.
-
- Bourne DE, Thompson M, Brody LL, Cotton M, Draper B, Laubscher R, et al. Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. Aids. 2009;23: 101–106. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

