Tropism of engineered and evolved recombinant AAV serotypes in the rd1 mouse and ex vivo primate retina
- PMID: 28872643
- PMCID: PMC5746594
- DOI: 10.1038/gt.2017.85
Tropism of engineered and evolved recombinant AAV serotypes in the rd1 mouse and ex vivo primate retina
Abstract
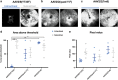
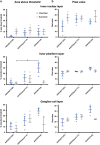
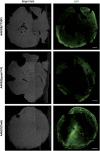
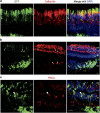
There is much debate on the adeno-associated virus (AAV) serotype that best targets specific retinal cell types and the route of surgical delivery-intravitreal or subretinal. This study compared three of the most efficacious AAV vectors known to date in a mouse model of retinal degeneration (rd1 mouse) and macaque and human retinal explants. Green fluorescent protein (GFP) driven by a ubiquitous promoter was packaged into three AAV capsids: AAV2/8(Y733F), AAV2/2(quad Y-F) and AAV2/2(7m8). Overall, AAV2/2(7m8) transduced the largest area of retina and resulted in the highest level of GFP expression, followed by AAV2/2(quad Y-F) and AAV2/8(Y733F). AAV2/2(7m8) and AAV2/2(quad Y-F) both resulted in similar patterns of transduction whether they were injected intravitreally or subretinally. AAV2/8(Y733F) transduced a significantly smaller area of retina when injected intravitreally compared with subretinally. Retinal ganglion cells, horizontal cells and retinal pigment epithelium expressed relatively high levels of GFP in the mouse retina, whereas amacrine cells expressed low levels of GFP and bipolar cells were infrequently transduced. Cone cells were the most frequently transduced cell type in macaque retina explants, whereas Müller cells were the predominant transduced cell type in human retinal explants. Of the AAV serotypes tested, AAV2/2(7m8) was the most effective at transducing a range of cell types in degenerate mouse retina and macaque and human retinal explants.
Conflict of interest statement
JGF is an inventor of a patent relating to AAV2/2(7m8). The other authors declare no conflict of interest.
Figures


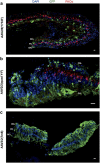
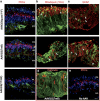
References
-
- Wu Y, Liang D, Wang Y, Bai M, Tang W, Bao S et al. Correction of a genetic disease in mouse via use of CRISPR-Cas9. Cell Stem Cell 2013; 13: 659–662. - PubMed

