Development of a direct contact astrocyte-human cerebral microvessel endothelial cells blood-brain barrier coculture model
- PMID: 28872681
- PMCID: PMC7938951
- DOI: 10.1111/jphp.12803
Development of a direct contact astrocyte-human cerebral microvessel endothelial cells blood-brain barrier coculture model
Abstract
Objectives: In conventional in-vitro blood-brain barrier (BBB) models, primary and immortalized brain microvessel endothelial cell (BMEC) lines are often cultured in a monolayer or indirect coculture or triculture configurations with astrocytes or pericytes, for screening permeation of therapeutic or potentially neurotoxic compounds. In each of these cases, the physiological relevancy associated with the direct contact between the BMECs, pericytes and astrocytes that form the BBB and resulting synergistic interactions are lost. We look to overcome this limitation with a direct contact coculture model.
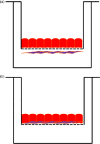
Methods: We established and optimized a direct interaction coculture system where primary human astrocytes are cultured on the apical surface of a Transwell® filter support and then human cerebral microvessel endothelial cells (hCMEC/D3) seeded directly on the astrocyte lawn.
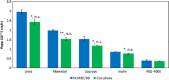
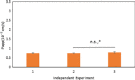
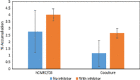
Key findings: The studies suggest the direct coculture model may provide a more restrictive and physiologically relevant model through a significant reduction in paracellular transport of model compounds in comparison with monoculture and indirect coculture. In comparison with existing methods, the indirect coculture and monoculture models utilized may limit cell-cell signaling between human astrocytes and BMECs that are possible with direct configurations.
Conclusions: Paracellular permeability reductions with the direct coculture system may enhance therapeutic agent and potential neurotoxicant screening for BBB permeability better than the currently available monoculture and indirect coculture in-vitro models.
Keywords: blood-brain barrier coculture; human astrocytes; human cerebral microvessel endothelial cells; paracellular permeability.
© 2017 Royal Pharmaceutical Society.
Figures



References
-
- Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 2013; 36: 437–449. - PubMed
-
- Ballabh P et al. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 2004; 16: 1–13. - PubMed
-
- Cecchelli R et al. Modelling of the blood-brain barrier in drug discovery and development. Nat Rev Drug Discov 2007; 6: 650–661. - PubMed
-
- Cohen-Kashi Malina K et al. Closing the gap between the in-vivo and in-vitro blood-brain barrier tightness. Brain Res 2009; 1284: 12–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

