A glutathione peroxidase from Antarctic psychrotrophic bacterium Pseudoalteromonas sp. ANT506: Cloning and heterologous expression of the gene and characterization of recombinant enzyme
- PMID: 28873004
- PMCID: PMC5736345
- DOI: 10.1080/21655979.2017.1373534
A glutathione peroxidase from Antarctic psychrotrophic bacterium Pseudoalteromonas sp. ANT506: Cloning and heterologous expression of the gene and characterization of recombinant enzyme
Abstract
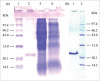
A glutathione peroxidase (GPx) gene, designated as PsGPx, was cloned from Antarctic psychrotrophic bacterium Pseudoalteromonas sp. ANT506 and expressed in Escherichia coli. The full-length PsGPx contained a 585-bp encoding 194 amino acids with predicted molecular masses of approx. 21.7 kDa. Multiple sequence alignments revealed that PsGPx belonged to the thioredoxin-like superfamily. PsGPx was heterologously overexpressed in E. coli, purified and characterized. The maximum catalytic temperature and pH value for recombinant PsGPx (rPsGPx) were 30°C and pH 9.0, respectively. rPsGPx retained 45% of the maximum activity at 0°C and exhibited high thermolability with a half-life of approx. 40 min at 40°C. In addition, the enzymatic activity of rPsGPx was still manifested under 3 M NaCl. The Km and Vmax values of the recombinant enzyme using GSH and H2O2 as substrates were 1.73 mM and 16.28 nmol/mL/min versus 2.46 mM and 21.50 nmol/mL/min, respectively.
Keywords: Antarctic sea ice; expression; gene; glutathione peroxidase; psychrotrophic.
Figures




References
-
- Mills G. Hemoglobin catabolism I. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative breakdown. J Biol Chem. 1957;229:189-197. PMID:13491573 - PubMed
-
- Valentina B, Marcus C, Giorgio C, Alessandro N, Slivia Q, Monica R, Antonella R, Stefano T, Fulvio U, Mattia Z, et al.. Protein disulfide isomerase and glutathione are alternative substrates in the one Cys catalytic cycle of glutathione peroxidase 7. Biochim Biophys Acta. 2013;1830:3846-3857. doi: 10.1016/j.bbagen.2013.02.017. PMID:23454490 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
