Identifying genetic variants that affect viability in large cohorts
- PMID: 28873088
- PMCID: PMC5584811
- DOI: 10.1371/journal.pbio.2002458
Identifying genetic variants that affect viability in large cohorts
Abstract
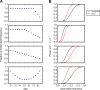
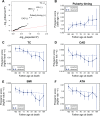
A number of open questions in human evolutionary genetics would become tractable if we were able to directly measure evolutionary fitness. As a step towards this goal, we developed a method to examine whether individual genetic variants, or sets of genetic variants, currently influence viability. The approach consists in testing whether the frequency of an allele varies across ages, accounting for variation in ancestry. We applied it to the Genetic Epidemiology Research on Adult Health and Aging (GERA) cohort and to the parents of participants in the UK Biobank. Across the genome, we found only a few common variants with large effects on age-specific mortality: tagging the APOE ε4 allele and near CHRNA3. These results suggest that when large, even late-onset effects are kept at low frequency by purifying selection. Testing viability effects of sets of genetic variants that jointly influence 1 of 42 traits, we detected a number of strong signals. In participants of the UK Biobank of British ancestry, we found that variants that delay puberty timing are associated with a longer parental life span (P~6.2 × 10-6 for fathers and P~2.0 × 10-3 for mothers), consistent with epidemiological studies. Similarly, variants associated with later age at first birth are associated with a longer maternal life span (P~1.4 × 10-3). Signals are also observed for variants influencing cholesterol levels, risk of coronary artery disease (CAD), body mass index, as well as risk of asthma. These signals exhibit consistent effects in the GERA cohort and among participants of the UK Biobank of non-British ancestry. We also found marked differences between males and females, most notably at the CHRNA3 locus, and variants associated with risk of CAD and cholesterol levels. Beyond our findings, the analysis serves as a proof of principle for how upcoming biomedical data sets can be used to learn about selection effects in contemporary humans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Sabeti PC, Schaffner SF, Fry B, Lohmueller J, Varilly P, Shamovsky O, et al. Positive natural selection in the human lineage. Science. 2006;312(5780):1614–1620. doi: 10.1126/science.1124309 - DOI - PubMed
-
- Nielsen R, Hellmann I, Hubisz M, Bustamante C, Clark AG. Recent and ongoing selection in the human genome. Nat Rev Genet. 2007;8(11):857–868. doi: 10.1038/nrg2187 - DOI - PMC - PubMed
-
- Fu W, Akey JM. Selection and adaptation in the human genome. Annu Rev Genomics Hum Genet. 2013;14:467–489. doi: 10.1146/annurev-genom-091212-153509 - DOI - PubMed
-
- Mathieson I, Lazaridis I, Rohland N, Mallick S, Patterson N, Roodenberg SA, et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature. 2015;528(7583):499–503. doi: 10.1038/nature16152 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

