Safety and Feasibility of Antiretroviral Preexposure Prophylaxis for Adolescent Men Who Have Sex With Men Aged 15 to 17 Years in the United States
- PMID: 28873128
- PMCID: PMC5710370
- DOI: 10.1001/jamapediatrics.2017.2007
Safety and Feasibility of Antiretroviral Preexposure Prophylaxis for Adolescent Men Who Have Sex With Men Aged 15 to 17 Years in the United States
Abstract
Importance: Adolescents represent a key population for implementing preexposure prophylaxis (PrEP) interventions worldwide, yet tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) for PrEP is only licensed for adults.
Objective: To examine the safety of and adherence to PrEP along with changes in sexual risk behavior among adolescent men who have sex with men (MSM).
Design, setting, and participants: Adolescent Medicine Trials Network for HIV/AIDS Interventions 113 (Project PrEPare) was a PrEP demonstration project that evaluated the safety, tolerability, and acceptability of TDF/FTC and patterns of use, rates of adherence, and patterns of sexual risk behavior among healthy young MSM aged 15 to 17 years. Participants were recruited from adolescent medicine clinics and their community partners in 6 US cities, had negative test results for human immunodeficiency virus (HIV) but were at high risk for acquiring an infection, and were willing to participate in a behavioral intervention and accept TDF/FTC as PrEP.
Exposures: All participants completed an individualized evidence-based behavioral intervention and were provided with daily TDF/FTC as PrEP for 48 weeks.
Main outcomes and measures: The main objectives were to: (1) provide additional safety data regarding TDF/FTC use among young MSM who had negative test results for HIV; (2) examine the acceptability, patterns of use, rates of adherence, and measured levels of tenofovir diphosphate in dried blood spots; and (3) examine patterns of risk behavior when young MSM were provided with a behavioral intervention in conjunction with open-label TDF/FTC.
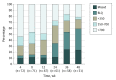
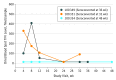
Results: Among 2864 individuals screened (from August 2013 to September 2014), 260 were eligible and 78 were enrolled (mean [SD] age, 16.5 [0.73] years), of whom 2 (3%) were Asian/Pacific Islander, 23 (29%) were black/African American, 11 (14%) were white, 16 (21%) were white Hispanic, and 26 (33%) were other/mixed race/ethnicity. Over 48 weeks of PrEP use, 23 sexually transmitted infections were diagnosed in 12 participants. The HIV seroconversion rate was 6.4 (95% CI: 1.3-18.7) per 100 person-years. Tenofovir diphosphate levels consistent with a high degree of anti-HIV protection (>700 fmol/punch) were found in 42 (54%), 37 (47%), 38 (49%), 22 (28%), 13 (17%), and 17 (22%) participants at weeks 4, 8, 12, 24, 36, and 48, respectively.
Conclusions and relevance: Adolescent Medicine Trials Network for HIV/AIDS Interventions 113 enrolled a diverse sample of adolescent MSM at risk for HIV who consented to study participation. Approximately half achieved protective drug levels during the monthly visits, but adherence decreased with quarterly visits. Youth may need additional contact with clinical staff members to maintain high adherence.
Trial registration: clinicaltrials.gov Identifier: NCT01769456.
Conflict of interest statement
Figures

Comment in
-
Human Immunodeficiency Virus Preexposure Prophylaxis for Adolescent Men: How Do We Ensure Health Equity for At-Risk Young Men?JAMA Pediatr. 2017 Nov 1;171(11):1041-1042. doi: 10.1001/jamapediatrics.2017.2397. JAMA Pediatr. 2017. PMID: 28873123 No abstract available.
-
Understanding the Possible Nonmedical Risks of Preexposure Prophylaxis Prescription in Adolescents-Reply.JAMA Pediatr. 2018 Apr 1;172(4):391-392. doi: 10.1001/jamapediatrics.2017.5599. JAMA Pediatr. 2018. PMID: 29435560 No abstract available.
-
Understanding the Possible Nonmedical Risks of Preexposure Prophylaxis Prescription in Adolescents.JAMA Pediatr. 2018 Apr 1;172(4):391. doi: 10.1001/jamapediatrics.2017.5596. JAMA Pediatr. 2018. PMID: 29435571 No abstract available.
References
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al. ; TDF2 Study Group . Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423-434. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

