N-terminally truncated POM121C inhibits HIV-1 replication
- PMID: 28873410
- PMCID: PMC5584925
- DOI: 10.1371/journal.pone.0182434
N-terminally truncated POM121C inhibits HIV-1 replication
Abstract
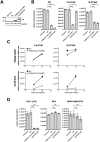
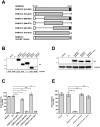
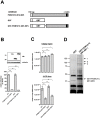
Recent studies have identified host cell factors that regulate early stages of HIV-1 infection including viral cDNA synthesis and orientation of the HIV-1 capsid (CA) core toward the nuclear envelope, but it remains unclear how viral DNA is imported through the nuclear pore and guided to the host chromosomal DNA. Here, we demonstrate that N-terminally truncated POM121C, a component of the nuclear pore complex, blocks HIV-1 infection. This truncated protein is predominantly localized in the cytoplasm, does not bind to CA, does not affect viral cDNA synthesis, reduces the formation of 2-LTR and diminished the amount of integrated proviral DNA. Studies with an HIV-1-murine leukemia virus (MLV) chimeric virus carrying the MLV-derived Gag revealed that Gag is a determinant of this inhibition. Intriguingly, mutational studies have revealed that the blockade by N-terminally-truncated POM121C is closely linked to its binding to importin-β/karyopherin subunit beta 1 (KPNB1). These results indicate that N-terminally-truncated POM121C inhibits HIV-1 infection after completion of reverse transcription and before integration, and suggest an important role for KPNB1 in HIV-1 replication.
Conflict of interest statement
Figures




References
-
- Goff SP. Host factors exploited by retroviruses. Nat Rev Microbiol. 2007;5(4):253–63. Epub 2007/02/28. doi: 10.1038/nrmicro1541 . - DOI - PubMed
-
- Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, Xavier RJ, et al. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319(5865):921–6. Epub 2008/01/12. doi: 10.1126/science.1152725 . - DOI - PubMed
-
- Hutten S, Walde S, Spillner C, Hauber J, Kehlenbach RH. The nuclear pore component Nup358 promotes transportin-dependent nuclear import. J Cell Sci. 2009;122(Pt 8):1100–10. Epub 2009/03/21. doi: 10.1242/jcs.040154 . - DOI - PubMed
-
- Woodward CL, Prakobwanakit S, Mosessian S, Chow SA. Integrase interacts with nucleoporin NUP153 to mediate the nuclear import of human immunodeficiency virus type 1. J Virol. 2009;83(13):6522–33. Epub 2009/04/17. doi: 10.1128/JVI.02061-08 ; - DOI - PMC - PubMed
-
- Ciuffi A, Bushman FD. Retroviral DNA integration: HIV and the role of LEDGF/p75. Trends Genet. 2006;22(7):388–95. doi: 10.1016/j.tig.2006.05.006 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

