Evaluation of robenidine analog NCL195 as a novel broad-spectrum antibacterial agent
- PMID: 28873428
- PMCID: PMC5584945
- DOI: 10.1371/journal.pone.0183457
Evaluation of robenidine analog NCL195 as a novel broad-spectrum antibacterial agent
Abstract
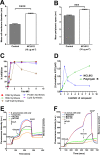
The spread of multidrug resistance among bacterial pathogens poses a serious threat to public health worldwide. Recent approaches towards combating antimicrobial resistance include repurposing old compounds with known safety and development pathways as new antibacterial classes with novel mechanisms of action. Here we show that an analog of the anticoccidial drug robenidine (4,6-bis(2-((E)-4-methylbenzylidene)hydrazinyl)pyrimidin-2-amine; NCL195) displays potent bactericidal activity against Streptococcus pneumoniae and Staphylococcus aureus by disrupting the cell membrane potential. NCL195 was less cytotoxic to mammalian cell lines than the parent compound, showed low metabolic degradation rates by human and mouse liver microsomes, and exhibited high plasma concentration and low plasma clearance rates in mice. NCL195 was bactericidal against Acinetobacter spp and Neisseria meningitidis and also demonstrated potent activity against A. baumannii, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae and Enterobacter spp. in the presence of sub-inhibitory concentrations of ethylenediaminetetraacetic acid (EDTA) and polymyxin B. These findings demonstrate that NCL195 represents a new chemical lead for further medicinal chemistry and pharmaceutical development to enhance potency, solubility and selectivity against serious bacterial pathogens.
Conflict of interest statement
Figures




References
-
- World Health Organization Media Centre; The top 10 causes of death: Fact sheet No 310. 2014.
-
- Murray CJ, Barber RM, Foreman KJ, Abbasoglu Ozgoren A, Abd-Allah F, Abera SF, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386(10009):2145–91. Epub 2015/09/01. doi: 10.1016/S0140-6736(15)61340-X ; PubMed Central PMCID: PMC4673910. - DOI - PMC - PubMed
-
- World Health Organization Media Centre; Pneumonia: Fact sheet No 331. 2015.
-
- Olarte L, Barson WJ, Barson RM, Lin PL, Romero JR, Tan TQ, et al. Impact of the 13-Valent Pneumococcal Conjugate Vaccine on Pneumococcal Meningitis in US Children. Clin Infect Dis. 2015;61(5):767–75. Epub 2015/05/15. doi: 10.1093/cid/civ368 . - DOI - PubMed
-
- Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378(9807):1962–73. Epub 2011/04/16. doi: 10.1016/S0140-6736(10)62225-8 ; PubMed Central PMCID: PMC3256741. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

