Deleterious effect of Usutu virus on human neural cells
- PMID: 28873445
- PMCID: PMC5600396
- DOI: 10.1371/journal.pntd.0005913
Deleterious effect of Usutu virus on human neural cells
Abstract
In the last decade, the number of emerging Flaviviruses described worldwide has increased considerably. Among them Zika virus (ZIKV) and Usutu virus (USUV) are African mosquito-borne viruses that recently emerged. Recently, ZIKV has been intensely studied due to major outbreaks associated with neonatal death and birth defects, as well as neurological symptoms. USUV pathogenesis remains largely unexplored, despite significant human and veterinary associated disorders. Circulation of USUV in Africa was documented more than 50 years ago, and it emerged in Europe two decades ago, causing massive bird mortality. More recently, USUV has been described to be associated with neurological disorders in humans such as encephalitis and meningoencephalitis, highlighting USUV as a potential health threat. The aim of this study was to evaluate the ability of USUV to infect neuronal cells. Our results indicate that USUV efficiently infects neurons, astrocytes, microglia and IPSc-derived human neuronal stem cells. When compared to ZIKV, USUV led to a higher infection rate, viral production, as well as stronger cell death and anti-viral response. Our results highlight the need to better characterize the physiopathology related to USUV infection in order to anticipate the potential threat of USUV emergence.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
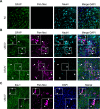
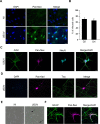
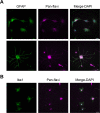
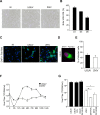
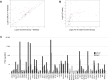
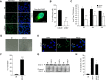
References
-
- Weissenböck H, Kolodziejek J, Url A, Lussy H, Rebel-Bauder B, Nowotny N. Emergence of Usutu virus, an African Mosquito-Borne Flavivirus of the Japanese Encephalitis Virus Group, Central Europe. Emerg Infect Dis. 2002;8: 652–656. doi: 10.3201/eid0807.020094 - DOI - PMC - PubMed
-
- Williams MC, Simpson DI, Haddow AJ, Knight EM. The isolation of West Nile virus from man and Usutu virus frome the bird-bitting mosquito mansonia aurites (theobald) in the entebee area of Uganda. Ann Trop Med Parasitol. 1964;58: 367–74. Available: http://www.ncbi.nlm.nih.gov/pubmed/14212897 - PubMed
-
- Weissenböck H, Bakonyi T, Chvala S, Nowotny N. Experimental Usutu virus infection of suckling mice causes neuronal and glial cell apoptosis and demyelination. Acta Neuropathol. 2004;108: 453–60. doi: 10.1007/s00401-004-0916-1 - DOI - PubMed
-
- Bakonyi T, Gould EA, Kolodziejek J, Weissenböck H, Nowotny N. Complete genome analysis and molecular characterization of Usutu virus that emerged in Austria in 2001: comparison with the South African strain SAAR-1776 and other flaviviruses. Virology. 2004;328: 301–10. doi: 10.1016/j.virol.2004.08.005 - DOI - PubMed
-
- Bakonyi T, Erdélyi K, Ursu K, Ferenczi E, Csörgo T, Lussy H, et al. Emergence of Usutu virus in Hungary. J Clin Microbiol. 2007;45: 3870–4. doi: 10.1128/JCM.01390-07 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

