A survey of the clinicopathological and molecular characteristics of patients with suspected Lynch syndrome in Latin America
- PMID: 28874130
- PMCID: PMC5586063
- DOI: 10.1186/s12885-017-3599-4
A survey of the clinicopathological and molecular characteristics of patients with suspected Lynch syndrome in Latin America
Abstract
Background: Genetic counselling and testing for Lynch syndrome (LS) have recently been introduced in several Latin America countries. We aimed to characterize the clinical, molecular and mismatch repair (MMR) variants spectrum of patients with suspected LS in Latin America.
Methods: Eleven LS hereditary cancer registries and 34 published LS databases were used to identify unrelated families that fulfilled the Amsterdam II (AMSII) criteria and/or the Bethesda guidelines or suggestive of a dominant colorectal (CRC) inheritance syndrome.
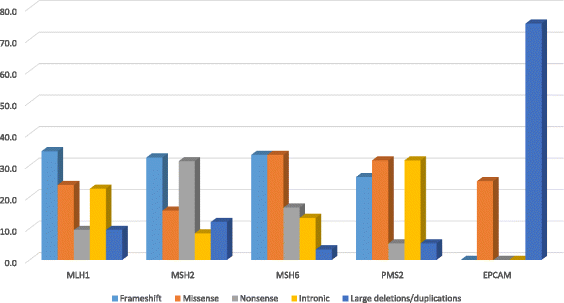
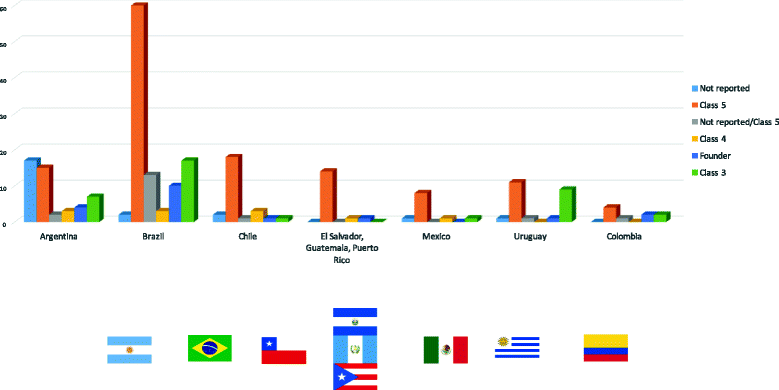
Results: We performed a thorough investigation of 15 countries and identified 6 countries where germline genetic testing for LS is available and 3 countries where tumor testing is used in the LS diagnosis. The spectrum of pathogenic MMR variants included MLH1 up to 54%, MSH2 up to 43%, MSH6 up to 10%, PMS2 up to 3% and EPCAM up to 0.8%. The Latin America MMR spectrum is broad with a total of 220 different variants which 80% were private and 20% were recurrent. Frequent regions included exons 11 of MLH1 (15%), exon 3 and 7 of MSH2 (17 and 15%, respectively), exon 4 of MSH6 (65%), exons 11 and 13 of PMS2 (31% and 23%, respectively). Sixteen international founder variants in MLH1, MSH2 and MSH6 were identified and 41 (19%) variants have not previously been reported, thus representing novel genetic variants in the MMR genes. The AMSII criteria was the most used clinical criteria to identify pathogenic MMR carriers although microsatellite instability, immunohistochemistry and family history are still the primary methods in several countries where no genetic testing for LS is available yet.
Conclusion: The Latin America LS pathogenic MMR variants spectrum included new variants, frequently altered genetic regions and potential founder effects, emphasizing the relevance implementing Lynch syndrome genetic testing and counseling in all of Latin America countries.
Keywords: Latin America; Lynch syndrome; Mmr; Variants.
Conflict of interest statement
Ethics approval and consent to participate
All patients provided an informed consent for inclusion into the Latin America registers during genetic counseling sessions and is in compliance with the Helsinki Declaration. Written informed consent was obtained from all participants during genetic counseling sessions.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Moller P, Seppala T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, Lindblom A, Macrae F, Blanco I, Sijmons R, et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut. 2015;66(3):464–72. - PMC - PubMed
-
- Moller P, Seppala T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, Lindblom A, Macrae F, Blanco I, Sijmons R, et al. Incidence of and survival after subsequent cancers in carriers of pathogenic MMR variants with previous cancer: a report from the prospective Lynch syndrome database. Gut. 2016; - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

