Sildenafil dosed concomitantly with bosentan for adult pulmonary arterial hypertension in a randomized controlled trial
- PMID: 28874133
- PMCID: PMC5586020
- DOI: 10.1186/s12872-017-0674-3
Sildenafil dosed concomitantly with bosentan for adult pulmonary arterial hypertension in a randomized controlled trial
Abstract
Background: Few controlled clinical trials exist to support oral combination therapy in pulmonary arterial hypertension (PAH).
Methods: Patients with PAH (idiopathic [IPAH] or associated with connective tissue disease [APAH-CTD]) taking bosentan (62.5 or 125 mg twice daily at a stable dose for ≥3 months) were randomized (1:1) to sildenafil (20 mg, 3 times daily; n = 50) or placebo (n = 53). The primary endpoint was change from baseline in 6-min walk distance (6MWD) at week 12, assessed using analysis of covariance. Patients could continue in a 52-week extension study. An analysis of covariance main-effects model was used, which included categorical terms for treatment, baseline 6MWD (<325 m; ≥325 m), and baseline aetiology; sensitivity analyses were subsequently performed.
Results: In sildenafil versus placebo arms, week-12 6MWD increases were similar (least squares mean difference [sildenafil-placebo], -2.4 m [90% CI: -21.8 to 17.1 m]; P = 0.6); mean ± SD changes from baseline were 26.4 ± 45.7 versus 11.8 ± 57.4 m, respectively, in IPAH (65% of population) and -18.3 ± 82.0 versus 17.5 ± 59.1 m in APAH-CTD (35% of population). One-year survival was 96%; patients maintained modest 6MWD improvements. Changes in WHO functional class and Borg dyspnoea score and incidence of clinical worsening did not differ. Headache, diarrhoea, and flushing were more common with sildenafil.
Conclusions: Sildenafil, in addition to stable (≥3 months) bosentan therapy, had no benefit over placebo for 12-week change from baseline in 6MWD. The influence of PAH aetiology warrants future study.
Trial registration: ClinicalTrials.gov NCT00323297 (registration date: May 5, 2006).
Keywords: Combination therapy; Sildenafil - Bosentan - pulmonary hypertension - randomized controlled trial -exercise test.
Conflict of interest statement
Authors’ information
Dr. Zhou was an employee of Pfizer Inc. at the time of writing.
Ethics approval and consent to participate
The coordinating ethics committee that approved the study was Comitato Etico Azienda Policlinico Umberto I (Universita’ degli Studi di Roma La Sapienza, Viale del Policlinico, 155, Roma 00155; reference number 1080/2006). At each of the 29 study centers, local institutional review boards or independent ethics committees additionally approved the trial protocol according to local and country-specific guidelines. A list of Independent Ethics Committees presented by country and site can be found in Additional file 1. Written informed consent was obtained from each patient.
Consent for publication
Not applicable.
Competing interests
Dr. Vizza has received fees for serving as a speaker, consultant, and advisory board member from Actelion, Dompè, GlaxoSmithKline, Italfarmaco, Lilly, Pfizer, and United Therapeutics. Dr. Jansa has received honoraria, consultancy fees and grants from Actelion Pharmaceuticals, Pfizer, Bayer, United Therapeutics and AOP Orphan Pharmaceuticals. Mr. Teal was employed by Pfizer at the time of this study; Dr. Dombi and Mr. Zhou are Pfizer employees.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
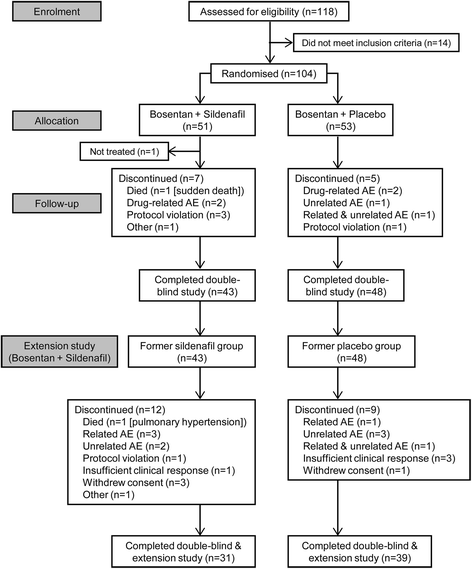

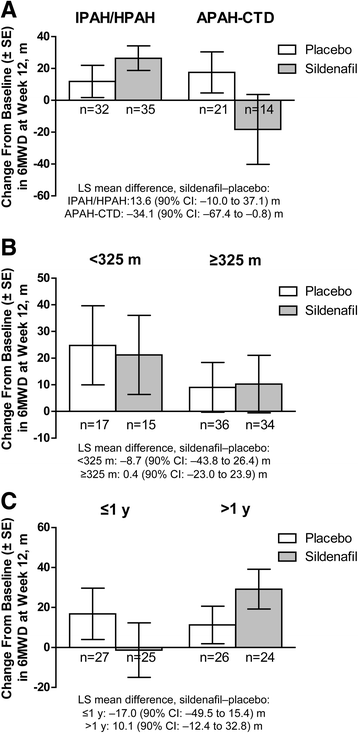
References
-
- Task Force for D. Treatment of Pulmonary Hypertension of European Society of C. European Respiratory S. International Society of H. Lung T, Galie N, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219–1263. doi: 10.1183/09031936.00139009. - DOI - PubMed
-
- McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation task force on expert consensus documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

