Evaluation of the Innate Immune Modulator Acitretin as a Strategy To Clear the HIV Reservoir
- PMID: 28874382
- PMCID: PMC5655051
- DOI: 10.1128/AAC.01368-17
Evaluation of the Innate Immune Modulator Acitretin as a Strategy To Clear the HIV Reservoir
Abstract
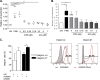
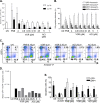
The persistence of HIV despite suppressive antiretroviral therapy is a major roadblock to HIV eradication. Current strategies focused on inducing the expression of latent HIV fail to clear the persistent reservoir, prompting the development of new approaches for killing HIV-positive cells. Recently, acitretin was proposed as a pharmacological enhancer of the innate cellular defense network that led to virus reactivation and preferential death of infected cells. We evaluated the capacity of acitretin to reactivate and/or to facilitate immune-mediated clearance of HIV-positive cells. Acitretin did not induce HIV reactivation in latently infected cell lines (J-Lat and ACH-2). We could observe only modest induction of HIV reactivation by acitretin in latently green fluorescent protein-HIV-infected Jurkat cells, comparable to suboptimal concentrations of vorinostat, a known latency-reversing agent (LRA). Acitretin induction was insignificant, however, compared to optimal concentrations of LRAs. Acitretin failed to reactivate HIV in a model of latently infected primary CD4+ T cells but induced retinoic acid-inducible gene I (RIG-I) and mitochondrial antiviral signaling (MAVS) expression in infected and uninfected cells, confirming the role of acitretin as an innate immune modulator. However, this effect was not associated with selective killing of HIV-positive cells. In conclusion, acitretin-mediated stimulation of the RIG-I pathway for HIV reactivation is modest and thus may not meaningfully affect the HIV reservoir. Stimulation of the RIG-I-dependent interferon (IFN) cascade by acitretin may not significantly affect the selective destruction of latently infected HIV-positive cells.
Keywords: HIV-1; RIG-I; cell death; interferons; latency; reactivation.
Copyright © 2017 American Society for Microbiology.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

