α-Catenin homodimers are recruited to phosphoinositide-activated membranes to promote adhesion
- PMID: 28874417
- PMCID: PMC5674881
- DOI: 10.1083/jcb.201612006
α-Catenin homodimers are recruited to phosphoinositide-activated membranes to promote adhesion
Abstract
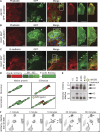
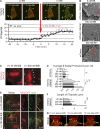
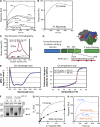
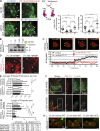
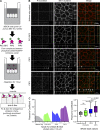
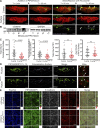
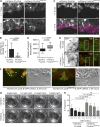
A unique feature of α-catenin localized outside the cadherin-catenin complex is its capacity to form homodimers, but the subcellular localization and functions of this form of α-catenin remain incompletely understood. We identified a cadherin-free form of α-catenin that is recruited to the leading edge of migrating cells in a phosphatidylinositol 3-kinase-dependent manner. Surface plasmon resonance analysis shows that α-catenin homodimers, but not monomers, selectively bind phosphatidylinositol-3,4,5-trisphosphate-containing lipid vesicles with high affinity, where three basic residues, K488, K493, and R496, contribute to binding. Chemical-induced dimerization of α-catenin containing a synthetic dimerization domain promotes its accumulation within lamellipodia and elaboration of protrusions with extended filopodia, which are attenuated in the α-cateninKKR<3A mutant. Cells restored with a full-length, natively homodimerizing form of α-cateninKKR<3A display reduced membrane recruitment, altered epithelial sheet migrations, and weaker cell-cell adhesion compared with WT α-catenin. These findings show that α-catenin homodimers are recruited to phosphoinositide-activated membranes to promote adhesion and migration, suggesting that phosphoinositide binding may be a defining feature of α-catenin function outside the cadherin-catenin complex.
© 2017 Wood et al.
Figures
Comment in
-
So far, yet so close: α-Catenin dimers help migrating cells get together.J Cell Biol. 2017 Nov 6;216(11):3437-3439. doi: 10.1083/jcb.201709056. Epub 2017 Oct 19. J Cell Biol. 2017. PMID: 29051263 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

