Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers
- PMID: 28874546
- PMCID: PMC5617273
- DOI: 10.1073/pnas.1704961114
Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers
Abstract
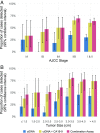
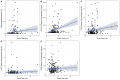
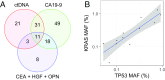
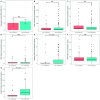
The earlier diagnosis of cancer is one of the keys to reducing cancer deaths in the future. Here we describe our efforts to develop a noninvasive blood test for the detection of pancreatic ductal adenocarcinoma. We combined blood tests for KRAS gene mutations with carefully thresholded protein biomarkers to determine whether the combination of these markers was superior to any single marker. The cohort tested included 221 patients with resectable pancreatic ductal adenocarcinomas and 182 control patients without known cancer. KRAS mutations were detected in the plasma of 66 patients (30%), and every mutation found in the plasma was identical to that subsequently found in the patient's primary tumor (100% concordance). The use of KRAS in conjunction with four thresholded protein biomarkers increased the sensitivity to 64%. Only one of the 182 plasma samples from the control cohort was positive for any of the DNA or protein biomarkers (99.5% specificity). This combinatorial approach may prove useful for the earlier detection of many cancer types.
Keywords: circulating tumor DNA; early cancer detection; liquid biopsy; pancreatic cancer; protein biomarkers.
Conflict of interest statement
Conflict of interest statement: C.M.S. and M.T.Y.-S. are founders and coowners of B9, Inc. and have no conflicts of interest with respect to the new technology described in this article. N.P., K.W.K., and B.V. are founders of Personal Genome Diagnostics, Inc. and PapGene, Inc. K.W.K. and B.V. are members of the Scientific Advisory Board of Sysmex-Inostics and Morphotek. B.V. is also a member of the Scientific Advisory Board of Exelixis GP. These companies and others have licensed technologies from Johns Hopkins, including those related to early diagnostics. N.P., K.W.K., and B.V. are the inventors of some of these technologies and receive equity or royalties from their licenses. The terms of these arrangements are being managed by the university in accordance with its conflict of interest policies. N.P., K.W.K., and B.V. have no conflicts of interest with respect to the new technology described in this article, as defined by the Johns Hopkins University policy on conflict of interest.
Figures

References
-
- Rahib L, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:2140–2141. - PubMed
-
- Ansari D, et al. Relationship between tumour size and outcome in pancreatic ductal adenocarcinoma. Br J Surg. 2017;104:600–607. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

