Active molecular iodine photochemistry in the Arctic
- PMID: 28874585
- PMCID: PMC5617258
- DOI: 10.1073/pnas.1702803114
Active molecular iodine photochemistry in the Arctic
Abstract
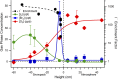
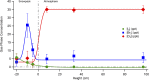
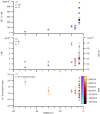
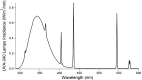
During springtime, the Arctic atmospheric boundary layer undergoes frequent rapid depletions in ozone and gaseous elemental mercury due to reactions with halogen atoms, influencing atmospheric composition and pollutant fate. Although bromine chemistry has been shown to initiate ozone depletion events, and it has long been hypothesized that iodine chemistry may contribute, no previous measurements of molecular iodine (I2) have been reported in the Arctic. Iodine chemistry also contributes to atmospheric new particle formation and therefore cloud properties and radiative forcing. Here we present Arctic atmospheric I2 and snowpack iodide (I-) measurements, which were conducted near Utqiaġvik, AK, in February 2014. Using chemical ionization mass spectrometry, I2 was observed in the atmosphere at mole ratios of 0.3-1.0 ppt, and in the snowpack interstitial air at mole ratios up to 22 ppt under natural sunlit conditions and up to 35 ppt when the snowpack surface was artificially irradiated, suggesting a photochemical production mechanism. Further, snow meltwater I- measurements showed enrichments of up to ∼1,900 times above the seawater ratio of I-/Na+, consistent with iodine activation and recycling. Modeling shows that observed I2 levels are able to significantly increase ozone depletion rates, while also producing iodine monoxide (IO) at levels recently observed in the Arctic. These results emphasize the significance of iodine chemistry and the role of snowpack photochemistry in Arctic atmospheric composition, and imply that I2 is likely a dominant source of iodine atoms in the Arctic.
Keywords: atmosphere; cryosphere; iodine; photochemistry; snowpack.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



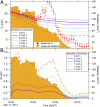
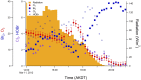


References
-
- Barrie LA, Bottenheim JW, Schnell RC, Crutzen PJ, Rasmussen RA. Ozone destruction and photochemical reactions at polar sunrise in the lower Arctic atmosphere. Nature. 1988;334:138–141.
-
- Oltmans SJ. Surface ozone measurements in clean air. J Geophys Res. 1981;86:1174–1180.
-
- Sturges WT, Barrie LA. Chlorine, bromine, and iodine in Arctic aerosols. Atmos Environ. 1988;22:1179–1194.
-
- Barrie LA, den Hartog G, Bottenheim JW, Landsberger S. Anthroprogenic aerosols and gases in the lower troposphere at Alert Canada in April 1986. J Atmos Chem. 1989;9:101–127.
-
- Liao J, et al. Observations of inorganic bromine (HOBr, BrO, and Br2) speciation at Barrow, Alaska, in spring 2009. J Geophys Res. 2012;117:D00R16.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

