FOXO1 deletion in keratinocytes improves diabetic wound healing through MMP9 regulation
- PMID: 28874756
- PMCID: PMC5585410
- DOI: 10.1038/s41598-017-10999-3
FOXO1 deletion in keratinocytes improves diabetic wound healing through MMP9 regulation
Abstract
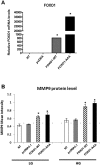
Keratinocyte migration is a key aspect of re-epithelialization during wound healing. Matrix metalloproteinase 9 (MMP9) contributes to this process and deficiencies in the MMP9 lead to impaired healing. Inappropriate expression of MMP9 also contributes to impaired re-epithelialization. Previously we demonstrated that FOXO1 was activated in wound healing but to higher levels in diabetic wounds. To address mechanisms of impaired re-epithelialization we examined MMP9 expression in vivo in full thickness dermal scalp wounds created in experimental K14.Cre + .Foxo1 L/L mice with lineage-specific Cre recombinase deletion of floxed FOXO1 and compared the results to control littermates. MMP9 was induced during wound healing but at a significantly higher level in diabetic compared to normal wounds. FOXO1 deletion substantially blocked this increase. By chromatin immunoprecipitation FOXO1 was shown to bind to the MMP9 promoter, FOXO1 overexpression increased MMP9 transcriptional activity and increased MMP9 expression stimulated by high glucose was blocked by FOXO1 deletion or FOXO1 knockdown. We also show for the first time that high glucose impairs keratinocyte migration by inducing high levels of MMP9 expression and establish that it involves FOXO1. Thus, FOXO1 drives high levels of MMP9 expression in diabetic wound healing, which represents a novel mechanism for impaired re-epithelization in diabetic wounds.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Ravanti L, Kahari VM. Matrix metalloproteinases in wound repair (review) INT J MOL MED. 2000;6:391–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

