Role of Smad3 and p38 Signalling in Cigarette Smoke-induced CFTR and BK dysfunction in Primary Human Bronchial Airway Epithelial Cells
- PMID: 28874823
- PMCID: PMC5585359
- DOI: 10.1038/s41598-017-11038-x
Role of Smad3 and p38 Signalling in Cigarette Smoke-induced CFTR and BK dysfunction in Primary Human Bronchial Airway Epithelial Cells
Abstract
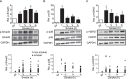
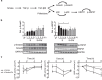
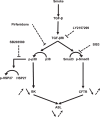
Mucociliary clearance (MCC) is a major airway host defence system that is impaired in patients with smoking-associated chronic bronchitis. This dysfunction is partially related to a decrease of airway surface liquid (ASL) volume that is in part regulated by apically expressed cystic fibrosis transmembrane conductance regulator (CFTR) and large-conductance, Ca2+-activated, and voltage dependent K+ (BK) channels. Here, data from human bronchial epithelial cells (HBEC) confirm that cigarette smoke not only downregulates CFTR activity but also inhibits BK channel function, thereby causing ASL depletion. Inhibition of signalling pathways involved in cigarette smoke-induced channel dysfunction reveals that CFTR activity is downregulated via Smad3 signalling whereas BK activity is decreased via the p38 cascade. In addition, pre-treatment with pirfenidone, a drug presently used to inhibit TGF-β signalling in idiopathic pulmonary fibrosis, ameliorated BK dysfunction and ASL volume loss. Taken together, our results highlight the importance of not only CFTR but also BK channel function in maintaining ASL homeostasis and emphasize the possibility that pirfenidone could be employed as a novel therapeutic regimen to help improve MCC in smoking-related chronic bronchitis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Therapeutic strategies to reverse cigarette smoke-induced ion channel and mucociliary dysfunction in COPD airway epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2025 Apr 1;328(4):L571-L585. doi: 10.1152/ajplung.00258.2024. Epub 2025 Mar 17. Am J Physiol Lung Cell Mol Physiol. 2025. PMID: 40095970 Free PMC article.
-
Transforming growth factor-β1 and cigarette smoke inhibit the ability of β2-agonists to enhance epithelial permeability.Am J Respir Cell Mol Biol. 2015 Jan;52(1):65-74. doi: 10.1165/rcmb.2013-0538OC. Am J Respir Cell Mol Biol. 2015. PMID: 24978189 Free PMC article.
-
The Cystic Fibrosis Transmembrane Conductance Regulator Potentiator Ivacaftor Augments Mucociliary Clearance Abrogating Cystic Fibrosis Transmembrane Conductance Regulator Inhibition by Cigarette Smoke.Am J Respir Cell Mol Biol. 2017 Jan;56(1):99-108. doi: 10.1165/rcmb.2016-0226OC. Am J Respir Cell Mol Biol. 2017. PMID: 27585394 Free PMC article.
-
Airway Hydration, Apical K(+) Secretion, and the Large-Conductance, Ca(2+)-activated and Voltage-dependent Potassium (BK) Channel.Ann Am Thorac Soc. 2016 Apr;13 Suppl 2(Suppl 2):S163-8. doi: 10.1513/AnnalsATS.201507-405KV. Ann Am Thorac Soc. 2016. PMID: 27115952 Free PMC article. Review.
-
Airway surface liquid homeostasis in cystic fibrosis: pathophysiology and therapeutic targets.Thorax. 2016 Mar;71(3):284-7. doi: 10.1136/thoraxjnl-2015-207588. Epub 2015 Dec 30. Thorax. 2016. PMID: 26719229 Review.
Cited by
-
30-Min Exposure to Tobacco Smoke Influences Airway Ion Transport-An In Vitro Study.Curr Oncol. 2023 Jul 22;30(7):7007-7018. doi: 10.3390/curroncol30070508. Curr Oncol. 2023. PMID: 37504368 Free PMC article.
-
The Effects of the Anti-aging Protein Klotho on Mucociliary Clearance.Front Med (Lausanne). 2020 Jan 24;6:339. doi: 10.3389/fmed.2019.00339. eCollection 2019. Front Med (Lausanne). 2020. PMID: 32039219 Free PMC article.
-
The effect of electronic cigarette and tobacco smoke exposure on COPD bronchial epithelial cell inflammatory responses.Int J Chron Obstruct Pulmon Dis. 2018 Mar 23;13:989-1000. doi: 10.2147/COPD.S157728. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 29615835 Free PMC article.
-
Vegetable glycerin e-cigarette aerosols cause airway inflammation and ion channel dysfunction.Front Pharmacol. 2022 Sep 26;13:1012723. doi: 10.3389/fphar.2022.1012723. eCollection 2022. Front Pharmacol. 2022. PMID: 36225570 Free PMC article.
-
Breathing in vitro: Designs and applications of engineered lung models.J Tissue Eng. 2021 Apr 28;12:20417314211008696. doi: 10.1177/20417314211008696. eCollection 2021 Jan-Dec. J Tissue Eng. 2021. PMID: 33996022 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

