Plant-expressed cocaine hydrolase variants of butyrylcholinesterase exhibit altered allosteric effects of cholinesterase activity and increased inhibitor sensitivity
- PMID: 28874829
- PMCID: PMC5585256
- DOI: 10.1038/s41598-017-10571-z
Plant-expressed cocaine hydrolase variants of butyrylcholinesterase exhibit altered allosteric effects of cholinesterase activity and increased inhibitor sensitivity
Erratum in
-
Author Correction: Plant-expressed cocaine hydrolase variants of butyrylcholinesterase exhibit altered allosteric effects of cholinesterase activity and increased inhibitor sensitivity.Sci Rep. 2018 Nov 16;8(1):17223. doi: 10.1038/s41598-018-35441-0. Sci Rep. 2018. PMID: 30443038 Free PMC article.
Abstract
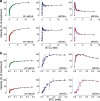
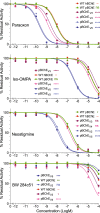
Butyrylcholinesterase (BChE) is an enzyme with broad substrate and ligand specificities and may function as a generalized bioscavenger by binding and/or hydrolyzing various xenobiotic agents and toxicants, many of which target the central and peripheral nervous systems. Variants of BChE were rationally designed to increase the enzyme's ability to hydrolyze the psychoactive enantiomer of cocaine. These variants were cloned, and then expressed using the magnICON transient expression system in plants and their enzymatic properties were investigated. In particular, we explored the effects that these site-directed mutations have over the enzyme kinetics with various substrates of BChE. We further compared the affinity of various anticholinesterases including organophosphorous nerve agents and pesticides toward these BChE variants relative to the wild type enzyme. In addition to serving as a therapy for cocaine addiction-related diseases, enhanced bioscavenging against other harmful agents could add to the practicality and versatility of the plant-derived recombinant enzyme as a multivalent therapeutic.
Conflict of interest statement
K.E.L., L.K., S.B., C.-G.Z. and T.S.M. are listed as inventors in various patents and patent applications relating to various aspects of the presented data.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

