Hyaluronic Acid Coated Chitosan Nanoparticles Reduced the Immunogenicity of the Formed Protein Corona
- PMID: 28874846
- PMCID: PMC5585335
- DOI: 10.1038/s41598-017-10836-7
Hyaluronic Acid Coated Chitosan Nanoparticles Reduced the Immunogenicity of the Formed Protein Corona
Abstract
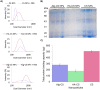
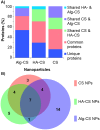
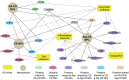
Studying the interactions of nanoparticles (NPs) with serum proteins is necessary for the rational development of nanocarriers. Optimum surface chemistry is a key consideration to modulate the formation of the serum protein corona (PC) and the resultant immune response. We investigated the constituent of the PC formed by hyaluronic acid-coated chitosan NPs (HA-CS NPs). Non-decorated chitosan NPs (CS NPs) and alginate-coated chitosan NPs (Alg-CS NPs) were utilized as controls. Results show that HA surface modifications significantly reduced protein adsorption relative to controls. Gene Ontology analysis demonstrates that HA-CS NPs were the least immunogenic nanocarriers. Indeed, less inflammatory proteins were adsorbed onto HA-CS NPs as opposed to CS and Alg-CS NPs. Interestingly, HA-CS NPs differentially adsorbed two unique anti-inflammatory proteins (ITIH4 and AGP), which were absent from the PC of both controls. On the other hand, CS and Alg-CS NPs selectively adsorbed a proinflammatory protein (Clusterin) that was not found on the surfaces of HA-CS NPs. While further studies are needed to investigate abilities of the PCs of only ITIH4 and AGP to modulate the interaction of NPs with the host immune system, our results suggest that this proof-of-concept could potentially be utilized to reduce the immunogenicity of a wide range of nanomaterials.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Lynch I, Dawson KA. Protein-nanoparticle interactions. Nano Today. 2008;3:40–47. doi: 10.1016/S1748-0132(08)70014-8. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

