Different Angiogenic Potentials of Mesenchymal Stem Cells Derived from Umbilical Artery, Umbilical Vein, and Wharton's Jelly
- PMID: 28874910
- PMCID: PMC5569878
- DOI: 10.1155/2017/3175748
Different Angiogenic Potentials of Mesenchymal Stem Cells Derived from Umbilical Artery, Umbilical Vein, and Wharton's Jelly
Abstract
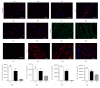
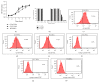
Human mesenchymal stem cells derived from the umbilical cord (UC) are a favorable source for allogeneic cell therapy. Here, we successfully isolated the stem cells derived from three different compartments of the human UC, including perivascular stem cells derived from umbilical arteries (UCA-PSCs), perivascular stem cells derived from umbilical vein (UCV-PSCs), and mesenchymal stem cells derived from Wharton's jelly (WJ-MSCs). These cells had the similar phenotype and differentiation potential toward adipocytes, osteoblasts, and neuron-like cells. However, UCA-PSCs and UCV-PSCs had more CD146+ cells than WJ-MSCs (P < 0.05). Tube formation assay in vitro showed the largest number of tube-like structures and branch points in UCA-PSCs among the three stem cells. Additionally, the total tube length in UCA-PSCs and UCV-PSCs was significantly longer than in WJ-MSCs (P < 0.01). Microarray, qRT-PCR, and Western blot analysis showed that UCA-PSCs had the highest expression of the Notch ligand Jagged1 (JAG1), which is crucial for blood vessel maturation. Knockdown of Jagged1 significantly impaired the angiogenesis in UCA-PSCs. In summary, UCA-PSCs are promising cell populations for clinical use in ischemic diseases.
Figures





References
-
- Carvalho M. M., Teixeira F. G., Reis R. L., Sousa N., Salgado A. J. Mesenchymal stem cells in the umbilical cord: phenotypic characterization, secretome and applications in central nervous system regenerative medicine. Current Stem Cell Research & Therapy. 2011;6(3):221–228. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

