Association of clinical trial enrollment and survival using contemporary therapy for pediatric acute lymphoblastic leukemia
- PMID: 28876513
- PMCID: PMC7553364
- DOI: 10.1002/pbc.26788
Association of clinical trial enrollment and survival using contemporary therapy for pediatric acute lymphoblastic leukemia
Abstract
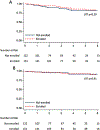
While early studies reported superior survival for cancer patients enrolled on clinical trials, recent findings are inconclusive. We investigated the association between enrollment on contemporary trials and event-free survival (EFS) in pediatric B-cell acute lymphoblastic leukemia (B-ALL). In a retrospective cohort of 274 children (1-21 years) treated for B-ALL from 2008 to 2015, 55.5% enrolled with no disparity in enrollment by age, sex, or ethnicity. Three-year EFS was similar for enrolled and not enrolled patients (90.1% [95% CI, 82.5-94.5] versus 86.5% [95% CI, 77.7-92.0]). Clinical trial enrollment did not affect pediatric B-ALL survival, albeit in a limited-size cohort treated at a single academic institution.
Keywords: clinical trial enrollment; clinical trial participation; disparities; pediatric B-cell acute lymphoblastic leukemia; survival.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Figures
References
-
- Vrooman LM, Stevenson KE, Supko JG, et al. Postinduction dexamethasone and individualized dosing of Escherichia coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study—Dana-Farber Cancer Institute ALL Consortium Protocol 00–01. J Clin Oncol. 2013;31(9):1202–1210. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources