Influence of Endo- and Exocyclic Heteroatoms on Stabilities and 1,3-Dipolar Cycloaddition Reactivities of Mesoionic Azomethine Ylides and Imines
- PMID: 28876936
- PMCID: PMC5679112
- DOI: 10.1021/acs.joc.7b01928
Influence of Endo- and Exocyclic Heteroatoms on Stabilities and 1,3-Dipolar Cycloaddition Reactivities of Mesoionic Azomethine Ylides and Imines
Abstract
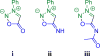
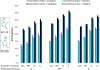
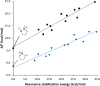
The geometries, stabilities, and 1,3-dipolar cycloaddition reactivities of 24 mesoionic azomethine ylides and imines were investigated using density functional theory calculations at the M06-2X/6-311+G-(d,p)/M06-2X/6-31G-(d) level. The computed structures highlight how the commonly used "aromatic" resonance form should be replaced by two more accurate resonance structures. Stabilities of the dipoles were assessed by various homodesmotic schemes and are consistent with these compounds being nonaromatic. The activation free energies with ethylene or acetylene range from 11.8 to 36.6 kcal/mol. Within each dipole type, the predicted cycloaddition reactivities correlate with the reaction energies and the resonance stabilization energies provided by the various substituents. Endocyclic (X) heteroatoms increase the reactivity of the 1,3-dipoles in the order of O > NH ≅ S, whereas exocyclic (Y) substituents increase it in the order of CH2 > NH > O > S. Distortion/interaction analysis indicated that the difference in reactivity between differently substituted 1,3-dipoles is driven by distortion, whereas the difference between azomethine ylides and imines is related to lower interaction energies of imines with the dipolarophiles.
Figures


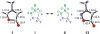






References
-
- Potts KT. Mesoionic Ring Systems. In: Padwa A, editor. 1,3-Dipolar Cycloaddition Chemistry. Vol. 2. Wiley; New York: 1984. pp. 1–82.
- Gribble GW. Mesoionic Ring Systems. In: Padwa A, Pearson WH, editors. The Chemistry of Heterocyclic Compounds: Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products. Vol. 59. John Wiley & Sons; Hoboken, NJ: 2002. pp. 681–753.
- Gingrich HL, Baum JL. Chemistry of Heterocyclic Compounds: Oxazoles. Vol. 45. Wiley; New York: 1986. Mesoionic Oxazoles; pp. 731–961.
- Gribble GW. Oxazoles: Synthesis, Reactions, and Spectroscopy, Part A. Vol. 60. Wiley; Hoboken, NJ: 2003. Mesoionic Oxazoles; pp. 473–576.
-
- Ollis WD, Ramsden CA. Adv. Heterocycl. Chem. 1976;19:1–122.
-
- Huisgen R, Gotthardt H, Bayer HO. Angew. Chem., Int. Ed. Engl. 1964;3:135–136.
- Huisgen R, Gotthardt H, Bayer HO, Schaefer FC. Angew. Chem., Int. Ed. Engl. 1964;3:136–137.
-
-
For a recent review, see: Reissig H-U, Zimmer R. Angew. Chem., Int. Ed. 2014;53:9708–9710.
-
-
- Earl JC, Mackney AW. J. Chem. Soc. 1935:899–900.
- Eade RA, Earl JC. J. Chem. Soc. 1946:591–593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

