Airway Mucin Concentration as a Marker of Chronic Bronchitis
- PMID: 28877023
- PMCID: PMC5706541
- DOI: 10.1056/NEJMoa1701632
Airway Mucin Concentration as a Marker of Chronic Bronchitis
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is characterized by chronic bronchitic and emphysematous components. In one biophysical model, the concentration of mucin on the airway surfaces is hypothesized to be a key variable that controls mucus transport in healthy persons versus cessation of transport in persons with muco-obstructive lung diseases. Under this model, it is postulated that a high mucin concentration produces the sputum and disease progression that are characteristic of chronic bronchitis.
Methods: We characterized the COPD status of 917 participants from the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) using questionnaires administered to participants, chest tomography, spirometry, and examination of induced sputum. Total mucin concentrations in sputum were measured with the use of size-exclusion chromatography and refractometry. In 148 of these participants, the respiratory secreted mucins MUC5AC and MUC5B were quantitated by means of mass spectrometry. Data from chronic-bronchitis questionnaires and data on total mucin concentrations in sputum were also analyzed in an independent 94-participant cohort.
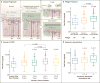
Results: Mean (±SE) total mucin concentrations were higher in current or former smokers with severe COPD than in controls who had never smoked (3166±402 vs. 1515±152 μg per milliliter) and were higher in participants with two or more respiratory exacerbations per year than in those with zero exacerbations (4194±878 vs. 2458±113 μg per milliliter). The absolute concentrations of MUC5B and MUC5AC in current or former smokers with severe COPD were approximately 3 times as high and 10 times as high, respectively, as in controls who had never smoked. Receiver-operating-characteristic curve analysis of the association between total mucin concentration and a diagnosis of chronic bronchitis yielded areas under the curve of 0.72 (95% confidence interval [CI], 0.65 to 0.79) for the SPIROMICS cohort and 0.82 (95% CI, 0.73 to 0.92) for the independent cohort.
Conclusions: Airway mucin concentrations may quantitate a key component of the chronic bronchitis pathophysiologic cascade that produces sputum and mediates disease severity. Studies designed to explore total mucin concentrations in sputum as a diagnostic biomarker and therapeutic target for chronic bronchitis appear to be warranted. (Funded by the National Heart, Lung, and Blood Institute and others.).
Figures



Comment in
-
Airway Mucins in Chronic Obstructive Pulmonary Disease.N Engl J Med. 2017 Sep 7;377(10):986-987. doi: 10.1056/NEJMe1707210. N Engl J Med. 2017. PMID: 28877010 No abstract available.
References
-
- Fletcher CM. The natural history of chronic bronchitis and emphysema: an eight-year study of early chronic obstructive lung disease in working men in London. Oxford, United Kingdom: Oxford University Press; 1976.
-
- Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Am J Respir Crit Care Med. 1996;153:1530–5. - PubMed
MeSH terms
Substances
Grants and funding
- HHSN268200900019C/HL/NHLBI NIH HHS/United States
- R01 HL103940/HL/NHLBI NIH HHS/United States
- P30 ES005605/ES/NIEHS NIH HHS/United States
- K24 HL137013/HL/NHLBI NIH HHS/United States
- HHSN268200900015C/HL/NHLBI NIH HHS/United States
- HHSN268200900016C/HL/NHLBI NIH HHS/United States
- R01 HL110906/HL/NHLBI NIH HHS/United States
- U01 HL137880/HL/NHLBI NIH HHS/United States
- HHSN268200900018C/HL/NHLBI NIH HHS/United States
- P30 DK054759/DK/NIDDK NIH HHS/United States
- HHSN268200900017C/HL/NHLBI NIH HHS/United States
- HHSN268200900020C/HL/NHLBI NIH HHS/United States
- HHSN268200900013C/HL/NHLBI NIH HHS/United States
- HHSN268200900014C/HL/NHLBI NIH HHS/United States
- P01 HL108808/HL/NHLBI NIH HHS/United States
- P50 HL120100/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
