Effectiveness of a Third Dose of MMR Vaccine for Mumps Outbreak Control
- PMID: 28877026
- PMCID: PMC6546095
- DOI: 10.1056/NEJMoa1703309
Effectiveness of a Third Dose of MMR Vaccine for Mumps Outbreak Control
Abstract
Background: The effect of a third dose of the measles-mumps-rubella (MMR) vaccine in stemming a mumps outbreak is unknown. During an outbreak among vaccinated students at the University of Iowa, health officials implemented a widespread MMR vaccine campaign. We evaluated the effectiveness of a third dose for outbreak control and assessed for waning immunity.
Methods: Of 20,496 university students who were enrolled during the 2015-2016 academic year, mumps was diagnosed in 259 students. We used Fisher's exact test to compare unadjusted attack rates according to dose status and years since receipt of the second MMR vaccine dose. We used multivariable time-dependent Cox regression models to evaluate vaccine effectiveness, according to dose status (three vs. two doses and two vs. no doses) after adjustment for the number of years since the second dose.
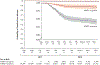
Results: Before the outbreak, 98.1% of the students had received at least two doses of MMR vaccine. During the outbreak, 4783 received a third dose. The attack rate was lower among the students who had received three doses than among those who had received two doses (6.7 vs. 14.5 cases per 1000 population, P<0.001). Students had more than nine times the risk of mumps if they had received the second MMR dose 13 years or more before the outbreak. At 28 days after vaccination, receipt of the third vaccine dose was associated with a 78.1% lower risk of mumps than receipt of a second dose (adjusted hazard ratio, 0.22; 95% confidence interval, 0.12 to 0.39). The vaccine effectiveness of two doses versus no doses was lower among students with more distant receipt of the second vaccine dose.
Conclusions: Students who had received a third dose of MMR vaccine had a lower risk of mumps than did those who had received two doses, after adjustment for the number of years since the second dose. Students who had received a second dose of MMR vaccine 13 years or more before the outbreak had an increased risk of mumps. These findings suggest that the campaign to administer a third dose of MMR vaccine improved mumps outbreak control and that waning immunity probably contributed to propagation of the outbreak. (Funded by the Centers for Disease Control and Prevention.).
Figures

Comment in
-
Third Dose of MMR Vaccine for Mumps Control.N Engl J Med. 2017 Dec 14;377(24):2403. doi: 10.1056/NEJMc1714219. N Engl J Med. 2017. PMID: 29236638 No abstract available.
-
Third Dose of MMR Vaccine for Mumps Control.N Engl J Med. 2017 Dec 14;377(24):2402. doi: 10.1056/NEJMc1714219. N Engl J Med. 2017. PMID: 29239592 No abstract available.
-
Third Dose of MMR Vaccine for Mumps Control.N Engl J Med. 2017 Dec 14;377(24):2402-3. doi: 10.1056/NEJMc1714219. N Engl J Med. 2017. PMID: 29239596 No abstract available.
-
Campaign Mode MMR Vaccination to Control Outbreak of Mumps in a Highly Vaccinated Population: Evidence-based Medicine viewpoint.Indian Pediatr. 2017 Dec 15;54(12):1047-1049. doi: 10.1007/s13312-017-1210-3. Indian Pediatr. 2017. PMID: 29317561 No abstract available.
-
Campaign Mode MMR Vaccination to Control Outbreak of Mumps in a Highly Vaccinated Population: Pediatrician's Viewpoint.Indian Pediatr. 2017 Dec 15;54(12):1049-1050. Indian Pediatr. 2017. PMID: 29317562 No abstract available.
-
Campaign Mode MMR Vaccination to Control Outbreak of Mumps in a Highly Vaccinated Population: Virologist's viewpoint.Indian Pediatr. 2017 Dec 15;54(12):1050-1051. Indian Pediatr. 2017. PMID: 29317563 No abstract available.
References
-
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Re- comm Rep 2013;62(RR-04):1–34. - PubMed
-
- Barskey AE, Glasser JW, LeBaron CW. Mumps resurgences in the United States: a historical perspective on unexpected elements. Vaccine 2009;27:6186–95. - PubMed
-
- Barskey AE, Schulte C, Rosen JB, et al. Mumps outbreak in Orthodox Jewish communities in the United States. N Engl J Med 2012;367:1704–13. - PubMed
-
- Morbidity and Mortality Weekly Report (MMWR) notifiable diseases and mortality tables (http://www.cdc.gov/mmwr/publications/index.html).
-
- Albertson JP, Clegg WJ, Reid HD, et al. Mumps outbreak at a university and recommendation for a third dose of measles– mumps–rubella vaccine — Illinois, 2015–2016. MMWR Morb Mortal Wkly Rep 2016; 65:731–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
