HPV-related Multiphenotypic Sinonasal Carcinoma: An Expanded Series of 49 Cases of the Tumor Formerly Known as HPV-related Carcinoma With Adenoid Cystic Carcinoma-like Features
- PMID: 28877065
- PMCID: PMC5680105
- DOI: 10.1097/PAS.0000000000000944
HPV-related Multiphenotypic Sinonasal Carcinoma: An Expanded Series of 49 Cases of the Tumor Formerly Known as HPV-related Carcinoma With Adenoid Cystic Carcinoma-like Features
Abstract
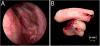
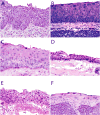
Human papillomavirus (HPV)-related multiphenotypic sinonasal carcinoma (HMSC), originally known as HPV-related carcinoma with adenoid cystic carcinoma-like features, is a peculiar neoplasm that is restricted to the sinonasal tract, exhibits features of both a surface-derived and salivary gland carcinoma (particularly adenoid cystic carcinoma), and is associated with high-risk HPV. Given the limited number of published cases, the full clinicopathologic spectrum of this neoplasm is unclear. Here, we present an updated experience of 49 cases. All cases of HMSC were obtained from the authors' files. Immunohistochemistry for p16, c-kit, and myoepithelial cell markers (S100, actin, calponin, p63, and/or p40) was performed along with RNA in situ hybridization for HPV (type 33-specific as well as a high-risk cocktail). Fluorescence in situ hybridization studies for fusions of MYB, NFIB, and MYBL1 was performed on a subset of cases. Clinical follow-up was obtained from medical records. A total of 49 cases of HMSC were collected. Twenty-eight (57%) were from women and 18 (43%) from men, ranging in age from 28 to 90 years (mean, 54 y). Of 40 cases with detailed staging information, 43% of HMSCs presented with a high T-stage (T3 or T4). Histologically, most grew predominantly as solid nests of basaloid cells exhibiting high mitotic rates and frequent necrosis, with histologic and immunohistochemical evidence of myoepithelial differentiation. Most cases also demonstrated foci of cribriform and/or tubular growth, along with an inconspicuous population of ducts. Thirty-four (69%) cases demonstrated an unusual pattern of surface involvement where markedly atypical squamous cells colonized tracts of the sinonasal mucosa. Less consistent histologic features included squamous differentiation within the invasive tumor (n=6), sarcomatoid transformation (n=5) including overt chondroid differentiation (n=3), and prominent epithelial-myoepithelial carcinoma-like growth (n=3). All cases were positive for p16 by immunostaining and HPV by RNA in situ hybridization. Thirty-three (67%) were positive for HPV 33. No cases tested for MYB, MYBL1, or NFIB gene fusions were positive. In the 38 cases with follow-up data, (mean follow-up, 42 mo) 14 recurred locally and 2 metastasized (lung, finger). There were no regional lymph node metastases, and no tumor-related deaths. HMSC is a distinct sinonasal neoplasm characterized by myoepithelial differentiation, frequent surface epithelial involvement, and the presence of high-risk HPV (especially type 33). Although it classically exhibits a cribriforming pattern that closely resembles adenoid cystic carcinoma, our expanded series highlights a histologic spectrum that is much broader than previously recognized, warranting a change in terminology. HMSC usually presents as a large and destructive sinonasal mass with high-grade histologic features, but it paradoxically behaves in a relatively indolent manner, underscoring the importance of distinguishing HMSC from true adenoid cystic carcinoma, squamous cell carcinoma, and other histologic mimickers.
Figures






References
-
- Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. Journal of the National Cancer Institute. 2000;92:709–720. - PubMed
-
- Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–2173. - PubMed
-
- El-Mofty SK, Lu DW. Prevalence of high-risk human papillomavirus DNA in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2005;29:1367–1372. - PubMed
-
- Alos L, Moyano S, Nadal A, et al. Human papillomaviruses are identified in a subgroup of sinonasal squamous cell carcinomas with favorable outcome. Cancer. 2009;115:2701–2709. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

