Surveillance for falsified and substandard medicines in Africa and Asia by local organizations using the low-cost GPHF Minilab
- PMID: 28877208
- PMCID: PMC5587284
- DOI: 10.1371/journal.pone.0184165
Surveillance for falsified and substandard medicines in Africa and Asia by local organizations using the low-cost GPHF Minilab
Abstract
Background: Substandard and falsified medical products present a serious threat to public health, especially in low- and middle-income countries. Their identification using pharmacopeial analysis is expensive and requires sophisticated equipment and highly trained personnel. Simple, low-cost technologies are required in addition to full pharmacopeial analysis in order to accomplish widespread routine surveillance for poor-quality medicines in low- and middle-income countries.
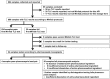
Methods: Ten faith-based drug supply organizations in seven countries of Africa and Asia were each equipped with a Minilab of the Global Pharma Health Fund (GPHF, Frankfurt, Germany), suitable for the analysis of about 85 different essential medicines by thin-layer chromatography. Each organization was asked to collect approximately 100 medicine samples from private local medicine outlets, especially from the informal sector. The medicine samples were tested locally according to the Minilab protocols. Medicines which failed Minilab testing were subjected to confirmatory analysis in a WHO-prequalified medicine quality control laboratory in Kenya.
Results: Out of 869 medicine samples, 21 were confirmed to be substandard or falsified medical products. Twelve did not contain the stated active pharmaceutical ingredient (API), six contained insufficient amounts of the API, and three showed insufficient dissolution of the API. The highest proportion of substandard and falsified medicines was found in Cameroon (7.1%), followed by the Democratic Republic of Congo (2.7%) and Nigeria (1.1%). Antimalarial medicines were most frequently found to be substandard or falsified (9.5% of all antimalarials). Thin-layer chromatography according to the Minilab protocols was found to be specific and reproducible in the identification of medicines which did not contain the stated API. Since only samples which failed Minilab testing were subjected to confirmatory testing using pharmacopeial methods, this study did not assess the sensitivity of the Minilab methodology in the detection of substandard medicines, and may underestimate the prevalence of poor-quality medicines.
Conclusions: Surveillance for poor-quality medicines can be carried out by local organizations in low- and middle-income countries using a simple, low-cost technology. Such surveillance can identify an important subgroup of the circulating substandard and falsified medical products and can help to prevent them from causing harm in patients. A collaboration of the national drug regulatory authorities with faith-based organizations and other NGOs may therefore represent a promising strategy towards the Sustainable Development Goal of "ensuring access to quality medicines".
Conflict of interest statement
Figures

References
-
- United Nations. General Assembly resolution 70/1. Transforming our world: the 2030 Agenda for Sustainable Development, A/RES/70/1 (25 September 2015). http://undocs.org/A/RES/70/1. Accessed 25 March 2017.
-
- World Health Organization. Member State mechanism on substandard/ spurious/falsely-labelled/falsified/counterfeit medical products. Appendix 3: Working definitions. WHO Excecutive Board 140th session, 10 January 2017. Provisional agenda item 8.6, EB140/23. http://apps.who.int/gb/e/e_eb140.html. Accessed 25 March 2017.
-
- Nayyar GM, Breman JG, Herrington JE. The global pandemic of falsified medicines: laboratory and field innovations and policy perspectives. Am J Trop Med Hyg. 2015;92(6 Suppl):2–7. Epub 2015/04/22. doi: 10.4269/ajtmh.15-0221 - DOI - PMC - PubMed
-
- Wirtz VJ, Hogerzeil HV, Gray AL, Bigdeli M, de Joncheere CP, Ewen MA, et al. Essential medicines for universal health coverage. Lancet. 2017;389:403–76. doi: 10.1016/S0140-6736(16)31599-9 - DOI - PMC - PubMed
-
- Nayyar GM, Breman JG, Newton PN, Herrington J. Poor-quality antimalarial drugs in southeast Asia and sub-Saharan Africa. Lancet Infect Dis. 2012;12(6):488–96. doi: 10.1016/S1473-3099(12)70064-6 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

