Functional characterization of T-cells from palatine tonsils in patients with chronic tonsillitis
- PMID: 28877231
- PMCID: PMC5587326
- DOI: 10.1371/journal.pone.0183214
Functional characterization of T-cells from palatine tonsils in patients with chronic tonsillitis
Abstract
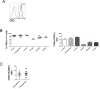
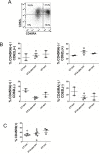
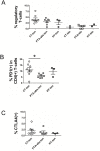
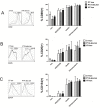
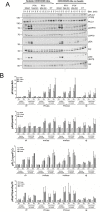
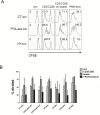
The palatine tonsils, localized in the oropharynx, are easily accessible secondary lymphoid tissue in humans. Inflammation of the palatine tonsils, local and chronic in case of chronic tonsillitis (CT) or acute in the presence of a peritonsillar abscess (PTA), ranks among the most common diseases in otolaryngology. However, the functionality of tonsillar immune cells, notably T-cells, in the context of these immune pathologies is poorly understood. We have examined the functional status of human tonsillar T-cells in CT and compared it to the acute inflammatory setting of a PTA. Patients presenting with CT (n = 10) or unilateral PTA (n = 7) underwent bilateral tonsillectomy and a subgroup of 8 patients underwent additional blood sampling. T-cells were purified via automated magnetic selection and subjected to flow cytometry-based immunophenotyping. In addition, the response to T-cell receptor (TCR) stimulation was assessed at the level of proximal signaling, activation marker expression and proliferation. We observed no difference between the percentage of T helper (CD4(+)) cells from tonsil tissue in CT and PTA, but observed a trend towards a higher percentage of T helper cells in the blood of patients with PTA versus CT, probably reflecting an acute, systemic bacterial infection in the former cohort. Tonsils from CT harbored more PD-1(+) CD4(+) T-cells, pointing to T-cell exhaustion due to chronic infection. This notion was supported by functional studies that showed a tendency to weaker TCR responses of tonsillar T-cells from CT. Intriguingly, tonsillar T-cells recurrently featured a dampened response to T-cell receptor stimulation at the level of receptor proximal signaling steps compared to peripheral T-cells. In sum, our study documents distinct differences in tonsillar T-cell class distribution and function between the various pathological conditions. Our observations are consistent with the concept that tonsillar T-cells react to infections by eliciting specific immunological responses in chronic versus acute settings of inflammation.
Conflict of interest statement
Figures

References
-
- Nave H, Gebert A, Pabst R. Morphology and immunology of the human palatine tonsil. Anat Embryol (Berl). 2001;204(5):367–73. - PubMed
-
- Olofsson K, Hellstrom S, Hammarstrom ML. The surface epithelium of recurrent infected palatine tonsils is rich in gammadelta T cells. Clin Exp Immunol. 1998;111(1):36–47. doi: 10.1046/j.1365-2249.1998.00446.x - DOI - PMC - PubMed
-
- Palomares O, Ruckert B, Jartti T, Kucuksezer UC, Puhakka T, Gomez E, et al. Induction and maintenance of allergen-specific FOXP3+ Treg cells in human tonsils as potential first-line organs of oral tolerance. J Allergy Clin Immunol. 2012;129(2):510–20, 20.e1-9. doi: 10.1016/j.jaci.2011.09.031 - DOI - PubMed
-
- Burton MJ, Glasziou PP. Tonsillectomy or adeno-tonsillectomy versus non-surgical treatment for chronic/recurrent acute tonsillitis. Cochrane Database Syst Rev. 2009(1):CD001802 doi: 10.1002/14651858.CD001802.pub2 - DOI - PubMed
-
- Paradise JL, Bluestone CD, Bachman RZ, Colborn DK, Bernard BS, Taylor FH, et al. Efficacy of tonsillectomy for recurrent throat infection in severely affected children. Results of parallel randomized and nonrandomized clinical trials. N Engl J Med. 1984;310(11):674–83. doi: 10.1056/NEJM198403153101102 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

