Cell-Intrinsic Glycogen Metabolism Supports Early Glycolytic Reprogramming Required for Dendritic Cell Immune Responses
- PMID: 28877459
- PMCID: PMC5657596
- DOI: 10.1016/j.cmet.2017.08.012
Cell-Intrinsic Glycogen Metabolism Supports Early Glycolytic Reprogramming Required for Dendritic Cell Immune Responses
Erratum in
-
Cell-Intrinsic Glycogen Metabolism Supports Early Glycolytic Reprogramming Required for Dendritic Cell Immune Responses.Cell Metab. 2019 Jul 2;30(1):225. doi: 10.1016/j.cmet.2019.05.017. Cell Metab. 2019. PMID: 31269426 Free PMC article. No abstract available.
Abstract
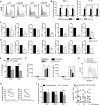
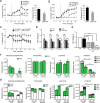
Dendritic cell (DC) activation by Toll-like receptor (TLR) agonists causes rapid glycolytic reprogramming that is required to meet the metabolic demands of their immune activation. Recent efforts in the field have identified an important role for extracellular glucose sourcing to support DC activation. However, the contributions of intracellular glucose stores to these processes have not been well characterized. We demonstrate that DCs possess intracellular glycogen stores and that cell-intrinsic glycogen metabolism supports the early effector functions of TLR-activated DCs. Inhibition of glycogenolysis significantly attenuates TLR-mediated DC maturation and impairs their ability to initiate lymphocyte activation. We further report that DCs exhibit functional compartmentalization of glucose- and glycogen-derived carbons, where these substrates preferentially contribute to distinct metabolic pathways. This work provides novel insights into nutrient homeostasis in DCs, demonstrating that differential utilization of glycogen and glucose metabolism regulates their optimal immune function.
Keywords: PYG; dendritic cells; glycogen; glycogen shunt; glycogenolysis; glycolysis.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Role of Cellular Metabolism in Pulmonary Diseases.Am J Respir Cell Mol Biol. 2018 Jul;59(1):127-129. doi: 10.1165/rcmb.2018-0103RO. Am J Respir Cell Mol Biol. 2018. PMID: 29634283 Free PMC article. No abstract available.
References
-
- Afzelius BA. Section staining for electron microscopy using tannic acid as a mordant: a simple method for visualization of glycogen and collagen. Microsc Res Tech. 1992;21:65–72. - PubMed
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. - PubMed
-
- Amati L, Pepe M, Passeri ME, Mastronardi ML, Jirillo E, Covelli V. Toll-like receptor signaling mechanisms involved in dendritic cell activation: potential therapeutic control of T cell polarization. Curr Pharm Des. 2006;12:4247–4254. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

