A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome
- PMID: 28877748
- PMCID: PMC5588692
- DOI: 10.1186/s13054-017-1823-x
A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome
Abstract
Background: Renin-angiotensin system (RAS) signaling and angiotensin-converting enzyme 2 (ACE2) have been implicated in the pathogenesis of acute respiratory distress syndrome (ARDS). We postulated that repleting ACE2 using GSK2586881, a recombinant form of human angiotensin-converting enzyme 2 (rhACE2), could attenuate acute lung injury.
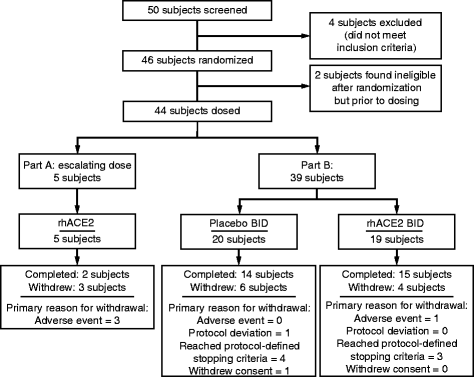
Methods: We conducted a two-part phase II trial comprising an open-label intrapatient dose escalation and a randomized, double-blind, placebo-controlled phase in ten intensive care units in North America. Patients were between the ages of 18 and 80 years, had an American-European Consensus Criteria consensus diagnosis of ARDS, and had been mechanically ventilated for less than 72 h. In part A, open-label GSK2586881 was administered at doses from 0.1 mg/kg to 0.8 mg/kg to assess safety, pharmacokinetics, and pharmacodynamics. Following review of data from part A, a randomized, double-blind, placebo-controlled investigation of twice-daily doses of GSK2586881 (0.4 mg/kg) for 3 days was conducted (part B). Biomarkers, physiological assessments, and clinical endpoints were collected over the dosing period and during follow-up.
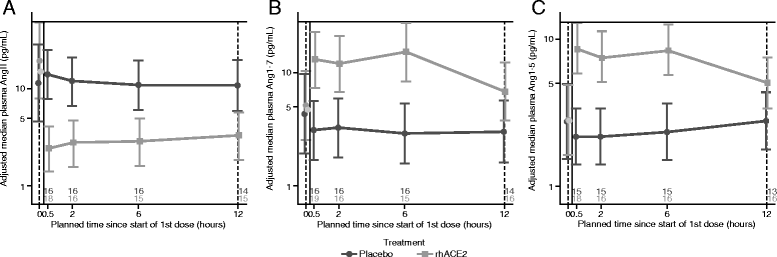
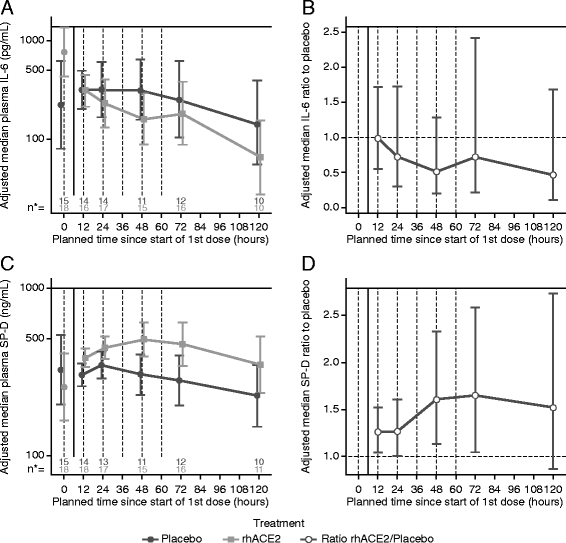
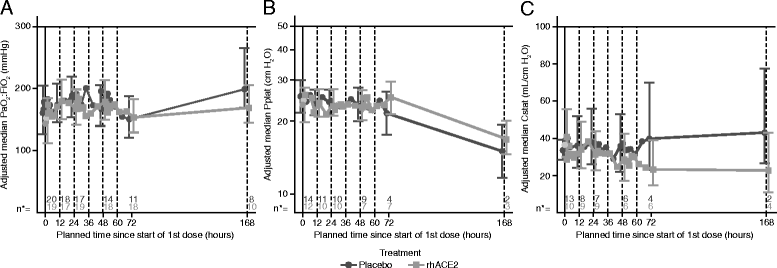
Results: Dose escalation in part A was well-tolerated without clinically significant hemodynamic changes. Part B was terminated after 39 of the planned 60 patients following a planned futility analysis. Angiotensin II levels decreased rapidly following infusion of GSK2586881, whereas angiotensin-(1-7) and angiotensin-(1-5) levels increased and remained elevated for 48 h. Surfactant protein D concentrations were increased, whereas there was a trend for a decrease in interleukin-6 concentrations in rhACE2-treated subjects compared with placebo. No significant differences were noted in ratio of partial pressure of arterial oxygen to fraction of inspired oxygen, oxygenation index, or Sequential Organ Failure Assessment score.
Conclusions: GSK2586881 was well-tolerated in patients with ARDS, and the rapid modulation of RAS peptides suggests target engagement, although the study was not powered to detect changes in acute physiology or clinical outcomes.
Trial registration: ClinicalTrials.gov, NCT01597635 . Registered on 26 January 2012.
Keywords: Acute lung injury; Acute respiratory failure; Adult; Angiotensin-converting enzyme 2; Humans; Interleukin-6; Renin-angiotensin system; Respiratory distress syndrome.
Conflict of interest statement
Authors’ information
Not applicable.
Ethics approval and consent to participate
After institutional review board approval was provided at each institution (available as part of Additional file 2), written informed consent was obtained from each patient or the patient’s legally authorized surrogate prior to conduct of study-specific procedures.
Consent for publication
Not applicable.
Competing interests
AK has received funding from GlaxoSmithKline, AstraZeneca, United Therapeutics, and Actelion Pharmaceuticals to conduct industry-sponsored research studies. TEA has received funding from GlaxoSmithKline to conduct industry-sponsored research studies. JDC has received institutional funding from GlaxoSmithKline and Bristol-Myers Squibb to conduct investigator-initiated observational studies. RH has received funding from GlaxoSmithKline, Asahi Kasei Pharma, La Jolla Pharmaceutical, Eli Lilly, AstraZeneca, and Pfizer to conduct industry-sponsored research studies. KH, WMP, TJW, SKS, DAF, DAL, AIB, and ALL are employees of GSK and own shares in the company. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Comment in
-
The evolving role of the renin-angiotensin system in ARDS.Crit Care. 2017 Dec 28;21(1):329. doi: 10.1186/s13054-017-1917-5. Crit Care. 2017. PMID: 29284527 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

