Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge
- PMID: 28878010
- PMCID: PMC5755978
- DOI: 10.1126/scitranslmed.aal1321
Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge
Abstract
Neutralizing antibodies to the V2 apex antigenic region of the HIV-1 envelope (Env) trimer are among the most prevalent cross-reactive antibodies elicited by natural infection. Two recently described V2-specific antibodies, PGDM1400 and CAP256-VRC26.25, have demonstrated exquisite potency and neutralization breadth against HIV-1. However, little data exist on the protective efficacy of V2-specific neutralizing antibodies. We created a novel SHIV-325c viral stock that included a clade C HIV-1 envelope and was susceptible to neutralization by both of these antibodies. Rhesus macaques received a single infusion of either antibody at three different concentrations (2, 0.4, and 0.08 mg/kg) before challenge with SHIV-325c. PGDM1400 was fully protective at the 0.4 mg/kg dose, whereas CAP256-VRC26.25-LS was fully protective even at the 0.08 mg/kg dose, which correlated with its greater in vitro neutralization potency against the challenge virus. Serum antibody concentrations required for protection were <0.75 μg/ml for CAP256-VRC26.25-LS. These data demonstrate unprecedented potency and protective efficacy of V2-specific neutralizing antibodies in nonhuman primates and validate V2 as a potential target for the prevention of HIV-1 infection in passive immunization strategies in humans.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures

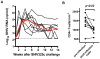
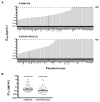



 ), that was not present in the challenge molecular clone or developed in any other animal.
), that was not present in the challenge molecular clone or developed in any other animal.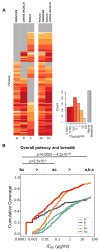
Comment in
-
Anti-HIV Passive Immunization: New Weapons in the Arsenal.Trends Microbiol. 2017 Dec;25(12):954-956. doi: 10.1016/j.tim.2017.10.006. Epub 2017 Oct 30. Trends Microbiol. 2017. PMID: 29097089 Free PMC article.
References
-
- Euler Z, van Gils MJ, Bunnik EM, Phung P, Schweighardt B, Wrin T, et al. Cross-reactive neutralizing humoral immunity does not protect from HIV type 1 disease progression. The Journal of infectious diseases. 2010;201(7):1045–53. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

