Structural basis for pH-insensitive inhibition of immunoglobulin G recycling by an anti-neonatal Fc receptor antibody
- PMID: 28878017
- PMCID: PMC5655520
- DOI: 10.1074/jbc.M117.807396
Structural basis for pH-insensitive inhibition of immunoglobulin G recycling by an anti-neonatal Fc receptor antibody
Abstract
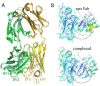
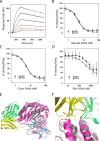
The neonatal Fc receptor FcRn plays a critical role in the trafficking of IgGs across tissue barriers and in retaining high circulating concentrations of both IgG and albumin. Although generally beneficial from an immunological perspective in maintaining IgG populations, FcRn can contribute to the pathogenesis of autoimmune disorders when an abnormal immune response targets normal biological components. We previously described a monoclonal antibody (DX-2507) that binds to FcRn with high affinity at both neutral and acidic pH, prevents the simultaneous binding of IgG, and reduces circulating IgG levels in preclinical animal models. Here, we report a 2.5 Å resolution X-ray crystal structure of an FcRn-DX-2507 Fab complex, revealing a nearly complete overlap of the IgG-Fc binding site in FcRn by complementarity-determining regions in DX-2507. This overlap explains how DX-2507 blocks IgG binding to FcRn and thereby shortens IgG half-life by preventing IgGs from recycling back into circulation. Moreover, the complex structure explains how the DX-2507 interaction is pH-insensitive unlike normal Fc interactions and how serum albumin levels are unaffected by DX-2507 binding. These structural studies could inform antibody-based therapeutic approaches for limiting the effects of IgG-mediated autoimmune disease.
Keywords: Fc receptor; antibody; autoimmune disease; immunoglobulin G (IgG); inhibitor; receptor recycling.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
This investigation was supported by Dyax Corp., now a wholly owned subsidiary of Shire Pharmaceuticals. J. A. K., G. P. C., J. C., K. J. K., A. P. L., S. R. C., C. T., B. A. A., D. J. S., and A. E. N. were employees of Dyax at the time of the study. J. A. K. and D. J. S. are currently Shire employees
Figures




References
-
- Nixon A. E., Chen J., Sexton D. J., Muruganandam A., Bitonti A. J., Dumont J., Viswanathan M., Martik D., Wassaf D., Mezo A., Wood C. R., Biedenkapp J. C., and TenHoor C. (2015) Fully human monoclonal antibody inhibitors of the neonatal fc receptor reduce circulating IgG in non-human primates. Front. Immunol. 6, 176. - PMC - PubMed
-
- Simister N. E., and Mostov K. E. (1989) An Fc receptor structurally related to MHC class I antigens. Nature 337, 184–187 - PubMed
-
- Martin W. L., West A. P. Jr, Gan L., and Bjorkman P. J. (2001) Crystal structure at 2.8 Å of an FcRn/heterodimeric Fc complex: mechanism of pH-dependent binding. Mol. Cell 7, 867–877 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

