Human Cytomegalovirus Particles Treated with Specific Antibodies Induce Intrinsic and Adaptive but Not Innate Immune Responses
- PMID: 28878085
- PMCID: PMC5660505
- DOI: 10.1128/JVI.00678-17
Human Cytomegalovirus Particles Treated with Specific Antibodies Induce Intrinsic and Adaptive but Not Innate Immune Responses
Abstract
Human cytomegalovirus (HCMV) persistently infects 40% to 100% of the human population worldwide. Experimental and clinical evidence indicates that humoral immunity to HCMV plays an important role in restricting virus dissemination and protecting the infected host from disease. Specific immunoglobulin preparations from pooled plasma of adults selected for high titers of HCMV antibodies have been used for the prevention of CMV disease in transplant recipients and pregnant women. Even though incubation of HCMV particles with these preparations leads to the neutralization of viral infectivity, it is still unclear whether the antibody-treated HCMV particles (referred to here as HCMV-Ab) enter the cells and modulate antiviral immune responses. Here we demonstrate that HCMV-Ab did enter macrophages. HCMV-Ab did not initiate the expression of immediate early antigens (IEAs) in macrophages, but they induced an antiviral state and rendered the cells less susceptible to HCMV infection upon challenge. Resistance to HCMV infection seemed to be due to the activation of intrinsic restriction factors and was independent of interferons. In contrast to actively infected cells, autologous NK cells did not degranulate against HCMV-Ab-treated macrophages, suggesting that these cells may not be eliminated by innate effector cells. Interestingly, HCMV-Ab-treated macrophages stimulated the proliferation of autologous adaptive CD4+ and CD8+ T cells. Our findings not only expand the current knowledge on virus-antibody immunity but may also be relevant for future vaccination strategies.IMPORTANCE Human cytomegalovirus (HCMV), a common herpesvirus, establishes benign but persistent infections in immunocompetent hosts. However, in subjects with an immature or dysfunctional immune system, HCMV is a major cause of morbidity and mortality. Passive immunization has been used in different clinical settings with variable clinical results. Intravenous hyperimmune globulin preparations (IVIg) are obtained from pooled adult human plasma selected for high anti-CMV antibody titers. While HCMV neutralization can be shown in vitro using different systems, data are lacking regarding the cross-influence of IVIg administration on the cellular immune responses. The aim of this study was to evaluate the effects of IVIg on distinct components of the immune response against HCMV, including antigen presentation by macrophages, degranulation of innate natural killer cells, and proliferation of adaptive CD4+ and CD8+ T cells.
Keywords: adaptive immunity; cytomegalovirus; innate immunity; intrinsic defenses; macrophages; neutralizing antibodies.
Copyright © 2017 American Society for Microbiology.
Figures
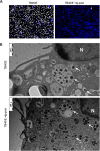
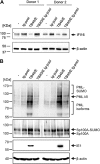


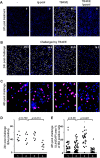


References
-
- Pass RF. 2001. Cytomegalovirus, p 2675–2705. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 4th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Macagno A, Bernasconi NL, Vanzetta F, Dander E, Sarasini A, Revello MG, Gerna G, Sallusto F, Lanzavecchia A. 2010. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J Virol 84:1005–1013. doi: 10.1128/JVI.01809-09. - DOI - PMC - PubMed
-
- Fouts AE, Chan P, Stephan JP, Vandlen R, Feierbach B. 2012. Antibodies against the gH/gL/UL128/UL130/UL131 complex comprise the majority of the anti-cytomegalovirus (anti-CMV) neutralizing antibody response in CMV hyperimmune globulin. J Virol 86:7444–7447. doi: 10.1128/JVI.00467-12. - DOI - PMC - PubMed
-
- Gerna G, Sarasini A, Patrone M, Percivalle E, Fiorina L, Campanini G, Gallina A, Baldanti F, Revello MG. 2008. Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. J Gen Virol 89:853–865. doi: 10.1099/vir.0.83523-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

