Wnt11 regulates cardiac chamber development and disease during perinatal maturation
- PMID: 28878122
- PMCID: PMC5621892
- DOI: 10.1172/jci.insight.94904
Wnt11 regulates cardiac chamber development and disease during perinatal maturation
Abstract
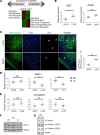
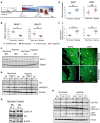
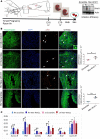
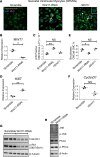
Ventricular chamber growth and development during perinatal circulatory transition is critical for functional adaptation of the heart. However, the chamber-specific programs of neonatal heart growth are poorly understood. We used integrated systems genomic and functional biology analyses of the perinatal chamber specific transcriptome and we identified Wnt11 as a prominent regulator of chamber-specific proliferation. Importantly, downregulation of Wnt11 expression was associated with cyanotic congenital heart defect (CHD) phenotypes and correlated with O2 saturation levels in hypoxemic infants with Tetralogy of Fallot (TOF). Perinatal hypoxia treatment in mice suppressed Wnt11 expression and induced myocyte proliferation more robustly in the right ventricle, modulating Rb1 protein activity. Wnt11 inactivation was sufficient to induce myocyte proliferation in perinatal mouse hearts and reduced Rb1 protein and phosphorylation in neonatal cardiomyocytes. Finally, downregulated Wnt11 in hypoxemic TOF infantile hearts was associated with Rb1 suppression and induction of proliferation markers. This study revealed a previously uncharacterized function of Wnt11-mediated signaling as an important player in programming the chamber-specific growth of the neonatal heart. This function influences the chamber-specific development and pathogenesis in response to hypoxia and cyanotic CHDs. Defining the underlying regulatory mechanism may yield chamber-specific therapies for infants born with CHDs.
Keywords: Cardiology; Cardiovascular disease; Cell cycle; Genetics; Molecular biology.
Conflict of interest statement
Figures




References
-
- Smolich JJ, Walker AM, Campbell GR, Adamson TM. Left and right ventricular myocardial morphometry in fetal, neonatal, and adult sheep. Am J Physiol. 1989;257(1 Pt 2):H1–H9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL108186/HL/NHLBI NIH HHS/United States
- R21 HL110667/HL/NHLBI NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- R01 HL143058/HL/NHLBI NIH HHS/United States
- R01 HG006264/HG/NHGRI NIH HHS/United States
- R01 HL070079/HL/NHLBI NIH HHS/United States
- K12 HD034610/HD/NICHD NIH HHS/United States
- R01 HL129639/HL/NHLBI NIH HHS/United States
- T90 DE022734/DE/NIDCR NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- R01 HL103205/HL/NHLBI NIH HHS/United States
- P30 NS062691/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

