Endothelium-derived extracellular vesicles promote splenic monocyte mobilization in myocardial infarction
- PMID: 28878126
- PMCID: PMC5621885
- DOI: 10.1172/jci.insight.93344
Endothelium-derived extracellular vesicles promote splenic monocyte mobilization in myocardial infarction
Abstract
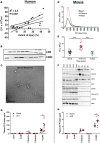
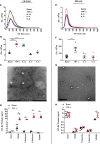
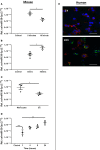
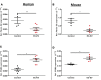
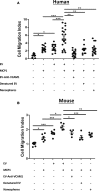
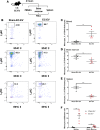
Transcriptionally activated monocytes are recruited to the heart after acute myocardial infarction (AMI). After AMI in mice and humans, the number of extracellular vesicles (EVs) increased acutely. In humans, EV number correlated closely with the extent of myocardial injury. We hypothesized that EVs mediate splenic monocyte mobilization and program transcription following AMI. Some plasma EVs bear endothelial cell (EC) integrins, and both proinflammatory stimulation of ECs and AMI significantly increased VCAM-1-positive EV release. Injected EC-EVs localized to the spleen and interacted with, and mobilized, splenic monocytes in otherwise naive, healthy animals. Analysis of human plasma EV-associated miRNA showed 12 markedly enriched miRNAs after AMI; functional enrichment analyses identified 1,869 putative mRNA targets, which regulate relevant cellular functions (e.g., proliferation and cell movement). Furthermore, gene ontology termed positive chemotaxis as the most enriched pathway for the miRNA-mRNA targets. Among the identified EV miRNAs, EC-associated miRNA-126-3p and -5p were highly regulated after AMI. miRNA-126-3p and -5p regulate cell adhesion- and chemotaxis-associated genes, including the negative regulator of cell motility, plexin-B2. EC-EV exposure significantly downregulated plexin-B2 mRNA in monocytes and upregulated motility integrin ITGB2. These findings identify EVs as a possible novel signaling pathway by linking ischemic myocardium with monocyte mobilization and transcriptional activation following AMI.
Keywords: Cardiology; Cell migration/adhesion; Monocytes; Vascular Biology; endothelial cells.
Conflict of interest statement
Figures
References
-
- Ruparelia N, et al. Acute myocardial infarction activates distinct inflammation and proliferation pathways in circulating monocytes, prior to recruitment, and identified through conserved transcriptional responses in mice and humans. Eur Heart J. 2015;36(29):1923–1934. doi: 10.1093/eurheartj/ehv195. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

